Hormonal Control of Regional Fat Distribution

The regional fat distribution in humans is clearly regulated by hormones, although genetic factors also play important roles. Not only sex steroid hormones are of importance, since adrenal corticosteroids also play a major role. This is seen clinically for example in Cushing's syndrome. In addition, peptide hormones such as insulin and growth hormone (GH) are important regulators of adipose tissue distribution, often on the basis of 'permissive' effects of the steroid hormones. In other words, steroid hormones provide a more long-term adaptation to permit the acute effects of peptide and catecholaminergic hormones.
 Adipose tissue metabolism is highly variable among species, human data often shows completely different results than on animal research.
Adipose tissue metabolism is highly variable among species, human data often shows completely different results than on animal research.
Professional and some recreative competing bodybuilders, use an enormous amount of peptides, insulin, thyroid hormones etc etc and also enormous amounts of food in the of-season, a practice known as “bulking”. This practice is often copied by newbies. They will certainly get bigger and stronger in a short time, but just watch the pictures, at the same time will store enormous amounts of fat also.
 They believe that they are also capable of trimming down their bodyfat again. Experienced bodybuilders do this when they are about to compete on stage again, this practice is known as “cutting”. But exactly “cutting” is extremely difficult, just like normal dieting. You are hungry weak and mostly loose muscle-mass just as fast as fat-mass!! These pro’s are pro’s because they know how to manipulate their hormones. Plus they use extremely dangerous compounds to control their appetite and loose fat. Compounds (like DNP) that can latterly cook someone not familiar with it.
They believe that they are also capable of trimming down their bodyfat again. Experienced bodybuilders do this when they are about to compete on stage again, this practice is known as “cutting”. But exactly “cutting” is extremely difficult, just like normal dieting. You are hungry weak and mostly loose muscle-mass just as fast as fat-mass!! These pro’s are pro’s because they know how to manipulate their hormones. Plus they use extremely dangerous compounds to control their appetite and loose fat. Compounds (like DNP) that can latterly cook someone not familiar with it.
Some strongman and powerlifters don’t mind a few extra pounds of fat, just look at the pictures in the top of this blogpost. But competing bodybuilders want an extreme low bodyfat not to mention the fluids that are excreted by the use of diuretics sodium control and controlled intake. Thus the stage ready Lee Priest, showed compared to his bulking picture, is unattainable for most of us will.
I know people want to start juicing as young as possible, but they should first be aware of how their body reacts to the different anabolic steroids and eventual low doses of GH. And not mess with thyroid hormones and insulin and IGF. Lots end with shrunken balls an low libido, but many more just get fat, once they stop cycling hormones. Why? because they don’t understand how hormones work and work synergistically or inhibiting to each other.
Hormones
Hormones exert powerful influences on body fat distribution in humans. Studies under fully controlled conditions in vitro (test-tube) have indicated that cortisol and insulin facilitate lipid accumulation by expressing lipoprotein lipase (LPL).
Growth hormone (GH) abolishes this and turns metabolism towards lipid mobilization. Testosterone and GH inhibit LPL and stimulate lipolysis markedly. Cortisol effects are mediated via a glucocorticoid receptor, and testosterone effects via an androgen receptor, the density of which appears to be higher in visceral than subcutaneous adipose tissue. The receptor-mediated effects are probably expressed via transcription of appropriate genes. The female sex steroids also regulate adipose tissue metabolism, but apparently not directly in the absence of specific cellular receptors. Oestrogens seem to exert net effects similar to those of testosterone. These results of cellular studies agree well with in vivo studies of triglyceride uptake and turnover in different adipose tissue regions. Furthermore, clinical entities with characteristic disturbances in hormone levels show the expected redistribution patterns.
In human adipose tissue the regulation of lipid accumulation at the level of the adipocyte is achieved mainly through the activity of lipoprotein lipase (LPL). The de-novo fatty acid synthesis from carbohydrate substrates is of considerably less quantitative importance. Lipid mobilization is regulated by the activity of the hormone-sensitive lipase, which is under the main, 'acute' control of catecholamines (stimulatory) and insulin (inhibitory) in human adipose tissue. There is also a possibility that under certain conditions an incomplete re-esterification of triglycerides may contribute to the mobilization of fatty acids, but information on this process is scarce, probably due to methodological difficulties.
Cellular studies Cortisol
Cortisol exerts major effects on adipose tissue metabolism, both on lipid accumulation and mobilization. In the presence of insulin, lipoprotein lipase (LPL) is markedly expressed. This expression is regulated by an interaction between transcription and a post-translational stabilizing effect. If GH is added, this expression is totally inhibited via a post-transcriptional effect which has so far not been identified. In relation to lipid mobilization, the addition of cortisol in the presence of insulin exerts slightly inhibitory effects. When GH is also added, activity is shifted dramatically to a lipid mobilizing effect .
These cortisol effects are mediated via a specific glucocorticoid receptor (GR), with a variable density in different regions of adipose tissue, in a ranking order of visceral > abdominal subcutaneous > femoral subcutaneous fat.
In summary, cortisol in the presence of insulin exerts powerful lipid accumulating effects, and these are abolished by GH which inhibits lipid accumulation and also activates lipid mobilization. These effects are probably most pronounced in visceral adipose tissue due to its high density of glucocorticoid receptors (GR).
Testosterone
On the lipid accumulating side, testosterone exerts inhibitory effects on LPL and glycerophosphate dehydrogenase, which are accentuated in the presence
of GH. This effect also occurs in the presence of cortisol. In other words, testosterone inhibits the LPL-activating effects of cortisol. Testosterone also regulates lipid mobilization in a powerful and multifaceted manner. Here testosterone and GH clearly have synergistic effects, since responses are much
less pronounced with each of the hormones alone
Graphic right
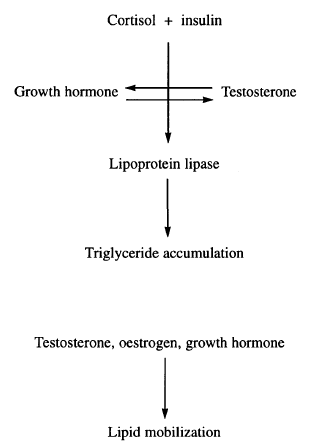 Overview of hormonal regulation of adipose tissue metabolism. Cortisol and insulin are the major lipid accumulating hormones. These effects are counteracted by sex steroid and growth hormones, which in addition facilitate lipid mobilization.
Overview of hormonal regulation of adipose tissue metabolism. Cortisol and insulin are the major lipid accumulating hormones. These effects are counteracted by sex steroid and growth hormones, which in addition facilitate lipid mobilization.
These effects are more pronounced in visceral tissue than in other fat depots due to a higher density of steroid hormone receptor. The regulatory steps affected seem to involve the lipolytic (3-adrenergic receptors and the cyclase, together with the protein kinase and/or hormone sensitive lipase. G-proteins do not seem to be affected.
The transcriptional effects of appropriate genes are also expressed via a specific androgen receptor (AR). This receptor is interesting because it is apparently autoregulated by its ligand testosterone, which seems to upregulate the density of the AR. In this way testosterone will amplify its own effects.
 The density of the androgen receptor is also apparently higher in visceral than subcutaneous adipose in the rat, and there is indirect evidence that this is also the case in humans. By analogy with cortisol, this would mean that androgen effects would be more pronounced in visceral than subcutaneous adipose tissues.
The density of the androgen receptor is also apparently higher in visceral than subcutaneous adipose in the rat, and there is indirect evidence that this is also the case in humans. By analogy with cortisol, this would mean that androgen effects would be more pronounced in visceral than subcutaneous adipose tissues.
The summary above concerns the influence of testosterone on male adipose tissue. Female adipose tissue also contains an androgen receptor, apparently identical to that in males as judged from its specificity and affinity determinations. It seems, however, that the effects of testosterone on female adipose tissue may differ from those in males.
The androgen receptor seems to be downregulated by oestrogen, suggesting that protection from androgen effects is provided by oestrogen. At the clinical experimental level, hyperandrogenic women tend to accumulate visceral fat, a phenomenon also witnessed after testosterone treatment of transsexual women .
 In summary, in male adipose tissue testosterone plus GH prevents lipid accumulation and stimulates lipid mobilization through an androgen receptor.
In summary, in male adipose tissue testosterone plus GH prevents lipid accumulation and stimulates lipid mobilization through an androgen receptor.
The density of this receptor seems to be upregulated by testosterone. This action is probably most pronounced in visceral fat where the androgen receptor density seems to be higher than in other regions. The net effect then would be to diminish the visceral fat depot mass, which has been detected clinically in men treated with testosterone, or with testosterone plus GH. The situation seems different in female adipose tissue where the net effects of testosterone seem to be the contrary, accumulation of visceral fat mass.
Oestrogen and progesterone
Studies of the cellular effects of these hormones have given inconclusive results. Direct effects in cell culture systems have not been demonstrated, and we have found no evidence for the presence of physiologically significant numbers of specific receptors in human adipose tissue. Nevertheless, systemic administration, particularly of oestrogen, no doubt exerts effects on metabolism, and distribution of adipose tissue. These observations suggest that indirect effects of these hormones occur in women, perhaps via an interference with the growth hormone secretion, regulation of AR density, or any other mechanisms.
Cortisol - Adrenalin - Insulin
 Cortisol is a neurotransmitter that comes into action after your body has created adrenalin. For the short term, you'll benefit from cortisol, but by an increase in stress in daily life, cortisol causes unhealthy situations. Cortisol creates more glucose in your blood, causing your blood sugar to rise. Then your body makes insulin, which brings the blood sugar to your body's cells. Cortisol causes the body to store the surpluses of blood sugars in your fat cells. One of the negative aspects of cortisol is that it can make you fat
Cortisol is a neurotransmitter that comes into action after your body has created adrenalin. For the short term, you'll benefit from cortisol, but by an increase in stress in daily life, cortisol causes unhealthy situations. Cortisol creates more glucose in your blood, causing your blood sugar to rise. Then your body makes insulin, which brings the blood sugar to your body's cells. Cortisol causes the body to store the surpluses of blood sugars in your fat cells. One of the negative aspects of cortisol is that it can make you fat
What you eat determines how much insulin your body produces. How much insulin your body produces determines your long-term health, but also your body weight (fat storage).
High insulin peaks after a very carbohydrate-rich meal or after eating very sugary products (or after an injection) are directly related to the production of the stress hormone cortisol (which, eventually causes fat deposits on your belly). If your body produces on several occasions much insulin because of the food you eat, then the body will produce more cortisol and adrenaline (the "degrading" hormone) to counter the constant rise of insulin (the "building effect '), trying to keep your body in balance.
But what cortisol also does is it breaks down muscle and therefore ensures that your resting metabolic rate (how much energy you burn at rest) decreases further.
And worse: too much adrenaline will eventually leads to even more insulin, because insulin eventually counteracts the adverse effects of adrenaline! These destructive interaction between insulin and cortisol / adrenalin forms the basis of a body which hormonally constantly out of balance (which often happens with 'yo-yo diets,' a lot of stress and a lot of carbohydrates / sugars) and therefore a body that is unhealthy on the inside and stores too much fat.
Remember that what you eat is more important than how much you eat. Always! Keep in mind that poor nutrition leads to stress in your body and that stress leads to increased body fat. It is important to be aware of what you eat.
Cortisol, in the presence of insulin, favours lipid accumulation in visceral depots. Testosterone and oestrogen have opposite effects. This opposition seems to be amplified by GH.
How does this fit with observations at the clinical level? We see that cortisol and insulin are coupled to lead to visceral fat accumulation while sex-specific steroid hormones in the presence of GH exert opposite effects. In fact, it seems likely that the balance between these two groups of opposing hormones actually determines the net outcome of body fat distribution to subcutaneous and visceral fat masses. This is illustrated by the following review of certain clinical conditions. Cushing's disease (high cortisol and insulin, low testosterone, oestrogen and GH) accumulates visceral fat, reversible by successful treatment.
 Ageing is often followed by low testosterone, oestrogen and GH but normal cortisol and insulin secretion, and visceral fat accumulation is a logical consequence. In the polycystic ovary syndrome, cortisol and insulin are elevated, and visceral fat accumulation seems to occur in spite of elevated androgens.
Ageing is often followed by low testosterone, oestrogen and GH but normal cortisol and insulin secretion, and visceral fat accumulation is a logical consequence. In the polycystic ovary syndrome, cortisol and insulin are elevated, and visceral fat accumulation seems to occur in spite of elevated androgens.
Smoking and alcohol are also characterized by larger than normal visceral depots, probably a consequence of a combination of elevated cortisol and low sex-specific steroid hormone secretions.
Finally, visceral obesity is characterized by elevated cortisol and insulin levels, and low sex steroid and GH secretions, which then probably provides a background to the elevation of visceral fat masses.
In summary, these clinical observations are in excellent agreement with the findings from studies at the cellular, experimental and interventional levels, indicating that the cortisol plus insulin couple directs storage fat to visceral depots, while the sex-specific steroid hormones and GH have opposite effects. The androgen effects on female adipose tissue are, however, unclear.



