SARMs are Steroids
 Just after the second world war a hopeful world produced a large new generation. Most of them did well. They have worked their whole life and are willing to pay for pharmaceutical aids that will prolong and ease their “golden years”. They want to grow old, but they want to grow old gracefully, maintaining their youthful skills. They don’t want to spent their last years in a wheelchair or in a nursing bed with a bedpan. They want to remain mobile and have a healthy sexlife. The success of Phizers Viagra proves that.
Just after the second world war a hopeful world produced a large new generation. Most of them did well. They have worked their whole life and are willing to pay for pharmaceutical aids that will prolong and ease their “golden years”. They want to grow old, but they want to grow old gracefully, maintaining their youthful skills. They don’t want to spent their last years in a wheelchair or in a nursing bed with a bedpan. They want to remain mobile and have a healthy sexlife. The success of Phizers Viagra proves that.
 Authorities all over the world are facing huge healthcare costs due to an aging babyboom. The steady loss of bone mass (osteoporosis) and of lean muscle mass (sarcopenia) that accompanies aging puts an increasing strain on our health care systems. It afflicts millions of aging people all over the world. Age-related loss of muscle mass, strength and frailty caused by declining levels of essential hormones in the body such as sex hormones and growth hormone stops them from carrying out everyday tasks and increases the risk of falling and eaking bones that are ittle because of osteoporosis. This is not only a major socio-economic as well as a major medical and financial problem, it is also important to improve the overall well-being of all these elderly. All this led to increased funds and need for scientific research towards prevention of age related diseases. We already knew that the exogenous administration of rhGH and sex-hormones such as Testosterone (HRT) could improve overall well-being in elderly.
Authorities all over the world are facing huge healthcare costs due to an aging babyboom. The steady loss of bone mass (osteoporosis) and of lean muscle mass (sarcopenia) that accompanies aging puts an increasing strain on our health care systems. It afflicts millions of aging people all over the world. Age-related loss of muscle mass, strength and frailty caused by declining levels of essential hormones in the body such as sex hormones and growth hormone stops them from carrying out everyday tasks and increases the risk of falling and eaking bones that are ittle because of osteoporosis. This is not only a major socio-economic as well as a major medical and financial problem, it is also important to improve the overall well-being of all these elderly. All this led to increased funds and need for scientific research towards prevention of age related diseases. We already knew that the exogenous administration of rhGH and sex-hormones such as Testosterone (HRT) could improve overall well-being in elderly.
Testosterone itself is naturally occurring and therefore not patentable. From most of the testosterone analogues the patent has long expired. But HRT/TRT and anti-aging is becoming a huge potential money maker. For the last decades the government, the supplement companies, big pharmaceutical companies and the medical community, demonized steroids as and evil drug that had horrifying side effects, because they rather sold patented drugs.
Because all these horrifying stories and the stigma of being Performance Enhancing Drugs (PEDs) aka Doping, anabolic androgenic steroids can’t put on the market as a “life saver.” That’s why developing a new structure away from the four carbon ring structure and a new fancy name
SARMs: Structure Activity Relationships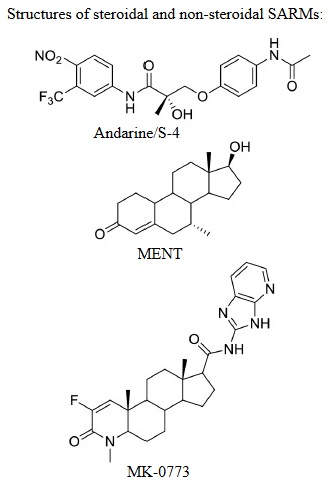
Structurally, SARMs can be categorized into steroidal and nonsteroidal SARMs. The steroidal SARMs are formed by modifying the chemical structure of testosterone molecule. Like MENT, that did not effectively maintain sperm suppression, necessary for male hormonal contraception recent animal models suggest that nonsteroidal SARMs may hold promise. The nonsteroidal SARMs are ligands that activate the androgenic receptor just like steroids, they just lack the four fused carbon ring structure. Technically speaking steroids in of themselves are selective for the androgenic receptor. And as you’ll see later there is only one receptor for Testosterone and all these other different anabolic androgenic steroids ..and the SARMs !!
For musclebuilding the goal is to maximize the anabolic effects of growth-promoting compounds, while reducing their androgenic effects. The term “anabolic” refers to the tissuebuilding properties of the compound, including increase in muscle mass (nitrogen retention) and decrease in bodyfat. The term “androgenic” refers to the increased development of male sexual characteristics by the compound, including behavioural changes and the development of the reproductive tract. Steroid derivatives have been synthesized with specific androgenic and anabolic activities which are determent by a bioassay using castrated rats. The effects of the compound on the myotropic activity in levator ani muscle is used as a measure of anabolic activity. The ratio of anabolic to androgenic activity is the Q value or anabolic index for the compound, a value greater than one indicates that the compound is primarily anabolic in nature. These Q values can vary amoung different laboratories due to non-standardized methods that are used in their determination. The levator ani muscle may also be an androgen-dependant muscle, so true measures of anabolic activity should be based on whole-body nitrogen retention studies.
 The structural features steroids that are related to anabolic versus androgenic activity are shown in the graphic left. Structure-activity studies have shown that the presence of the 3-keto group, the 5α-hydrogen and the 17β-hydroxyl increases the binding affinity of the steroid to the androgen receptor and the androgenic effects of steroids. Removal of the C19 (to form19-nor steroids) favours anabolic activity and reduces androgenic activity. Oxidation of the 17β-hydroxyl reduces androgenic activity and estrification of this hydroxyl group favours anabolic activity. Modification of the A ring by the junction with a pyrazole ring or oxygen atom at C2 leads to a considerable increase in anabolic activity. Anabolic activity can also be increased by alkylation at the 17α- or 7α-position.
The structural features steroids that are related to anabolic versus androgenic activity are shown in the graphic left. Structure-activity studies have shown that the presence of the 3-keto group, the 5α-hydrogen and the 17β-hydroxyl increases the binding affinity of the steroid to the androgen receptor and the androgenic effects of steroids. Removal of the C19 (to form19-nor steroids) favours anabolic activity and reduces androgenic activity. Oxidation of the 17β-hydroxyl reduces androgenic activity and estrification of this hydroxyl group favours anabolic activity. Modification of the A ring by the junction with a pyrazole ring or oxygen atom at C2 leads to a considerable increase in anabolic activity. Anabolic activity can also be increased by alkylation at the 17α- or 7α-position.
Due to poor oral bioavalability, potential hepatotoxity and, in particular, the lack of tissue selectivity of some steroids, a large number of non-steroidal androgenic compound, known as selective androgen receptor modulators (SARMs), have been developed. The earliest of these compounds developed were androgen antagonists, but agonist have also been produced.Ongoing research will lead to the development of SARMs with selective preferences for induvidual tissues or activities.
Mechanism of action
Androgens increase the retention of body protein, which can be the result of increased protein synthesis or decreased protein degradation. While testosteron increases protein synthesis, trenbolone promotes muscle growth by decreasing protein degradation.
Since the number of muscle fibers that someone has is fixed at birth or shortly thereafter subsequent muscle fie growth occurs by hypertrophy rather than hyperplasia. This requires an increase in the number of myonuclei, which come from satellite cells that fuse with the muscle fie. Androgen treatment causes a dose-dependent increase in the number of satellite cells.
Anabolic steroids can act by a direct mechanism through interaction or by indirect methods.
Indirect mechanisms include being aromatized to oestrogen and acting through the oestrogen receptor or modulating the production of other hormones, such as growth hormone, thyroid hormone and insulin.
 Direct effects
Direct effects
The direct effects of androgens are mediated by interaction with the androgen receptor. A large number of different androgenic compounds have been developed that are known as selective androgen receptor modulators (SARMs). However, only one androgen receptor has been identified and cloned.
The AR gene is located on the X chromosome and codes for a protein of 918 amino acids. As a member of the nuclear receptor family of transcription factors, it contains protein domains that can activate or repress activity. These domains are exposed upon hormone binding to allow interactions with various co-activators or co-repressors. These domains a re exposed upon hormone binding to allow interactions with various co-activators or co-repressors. The differential effects of androgens in various tissues may be due to differences in the levels of cofactors that affect the AR, or the presence of unique AR-interacting proteins such as ARIP-3, which is specific to testis. In addition, the binding of a particular SARM to the AR may cause a unique conformational change that exposes particular domains that allow the interaction of specific cofactors. This would lead to the selective regulation of individual genes in specific tissues by the AR. Ongoing research will lead to the development of SARMs with selective preferences for individual tissues or activities.
re exposed upon hormone binding to allow interactions with various co-activators or co-repressors. The differential effects of androgens in various tissues may be due to differences in the levels of cofactors that affect the AR, or the presence of unique AR-interacting proteins such as ARIP-3, which is specific to testis. In addition, the binding of a particular SARM to the AR may cause a unique conformational change that exposes particular domains that allow the interaction of specific cofactors. This would lead to the selective regulation of individual genes in specific tissues by the AR. Ongoing research will lead to the development of SARMs with selective preferences for individual tissues or activities.
Testosterone can unde rgo irreversible reduction by the 5α-reductase enzyme in some tissues to form 5α-dihydrotestosterone, which can be further metabolized to 17-keto steroids and polar metabolites that will have different binding characteristics to the AR. The aromatization of androgens with a Δ-4-3-keto group to oestrogens may also be important in some tissues, since oestrogen-like effects will be produced. The aromatase reaction can be blocked by removal of the C19 methyl function (as in nandrolone) or modification of the A ring (as in oxandrolone (left) and stanazol).
rgo irreversible reduction by the 5α-reductase enzyme in some tissues to form 5α-dihydrotestosterone, which can be further metabolized to 17-keto steroids and polar metabolites that will have different binding characteristics to the AR. The aromatization of androgens with a Δ-4-3-keto group to oestrogens may also be important in some tissues, since oestrogen-like effects will be produced. The aromatase reaction can be blocked by removal of the C19 methyl function (as in nandrolone) or modification of the A ring (as in oxandrolone (left) and stanazol).
Androgens have a primary effect on skeletal muscle through binding to specific receptors on the muscle. Because the activity of the 5α-reductase enzyme is low in skeletal muscle, testosterone is the major hormone for androgen action in muscle. Increased numbers of androgen receptors are found during the muscle hypertrophy seen with increased exercise or treatment with oxandrolone, which is a synthetic analogue of testosterone that cannot be aromatized to oestrogens. The number of free androgen receptors is higher in females and castrates compared to intact males. An oestrogen receptor has also been identified, and the free form of this receptor is present in higher amounts in males than in females. Steroids bind to receptors in the cytoplasm, and the hormone–receptor complex enters the nucleus to stimulate the transcription of hormonespecific genes. This increases the synthesis of myofiil and sarcoplasmic proteins to increase the overall muscle mass.
Indirect effects
Androgens can function indirectly by increasing the production of GH or IGF-I, and prior exposure to androgens can prime cells for the secondary actions of IGF-I. Glucocorticoids may increase the production of IGF-binding proteins to decrease IGF bioactivity. Anabolic steroids also reduce the production of LH and testosterone, delay sexual maturation and reduce testicular growth and spermatogenesis in men.
There appears to be a reciprocal relationship between testosterone (an anabolic hormone) and cortisol (a catabolic hormone) in men. Anabolic steroids could potentially act as antagonists of the catabolic action of glucocorticoids, by decreasing the concentration of the glucocorticoids, or by displacing the glucocorticoids from their receptors. Studies in vitro have shown that testosterone has a high affinity for the glucocorticoid receptor and could thus prevent the normal protein catabolic action of glucocorticoids.
There is some evidence that anabolic agents with oestrogenic activity increase the blood levels of insulin to promote growth. Oestrogens increase growth rate and food efficiency. Insulin has anabolic effects on adipose and skeletal muscle, while thyroid hormone (T3) has a catabolic effect on adipose tissue and an anabolic influence over skeletal muscle.
The term ‘anabolic’ refers to compounds that increase nitrogen retention. A number of different types of compounds, including insulin, growth hormone, β-agonists and steroids, exert anabolic effects.
Science:
Tissue selectivity of the anabolic steroid, 19-nor-4-androstenediol-3beta,17beta-diol in male Sprague Dawley rats: selective stimulation of muscle mass and bone mineral density relative to prostate mass. Page et all 2008:
“Stimulation of prostate growth is a major concern with testosterone therapy in older hypogonadal men. As a result, nonsteroidal selective androgen receptor modulators with anabolic activity but less prostate stimulation are being developed. Anabolic steroids might exhibit similar tissue selectivity. We hypothesized the anabolic steroid 19-nor-4-androstenediol-3beta,17beta-diol (3beta,19-NA) would increase muscle, lean body mass (LBM), and bone mineral density (BMD) with little stimulation of prostate growth.
In summary,3beta,19-NA increases muscle and bone mass without significant stimulation of prostate growth, suggesting it may have some properties of a steroidal selective androgen receptor modulator. Anabolic steroids such as 3beta,19-NA should be studied further to determine their mechanisms of tissue selectivity and effects in men.”
===========================================================================
Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity. Yarrow et all 2010:
“Recently, the development of selective androgen receptor modulators (SARMs) has been suggested as a means of combating the deleterious catabolic effects of hypogonadism, especially in skeletal muscle and bone, without inducing the undesirable androgenic effects (e.g., prostate enlargement and polycythemia) associated with testosterone administration. 17beta-Hydroxyestra-4,9,11-trien-3-one (trenbolone; 17beta-TBOH), a synthetic analog of testosterone, may be capable of inducing SARM-like effects as it binds to androgen receptors (ARs) with approximately three times the affinity of testosterone and has been shown to augment skeletal muscle mass and bone growth and reduce adiposity in a variety of mammalian species. In addition to its direct actions through ARs, 17beta-TBOH may also exert anabolic effects by altering the action of endogenous growth factors or inhibiting the action of glucocorticoids. Compared to testosterone, 17beta-TBOH appears to induce less growth in androgen-sensitive organs which highly express the 5alpha reductase enzyme (e.g., prostate tissue and accessory sex organs). The reduced androgenic effects result from the fact that 17beta-TBOH is metabolized to less potent androgens in vivo; while testosterone undergoes tissue-specific biotransformation to more potent steroids, dihydrotestosterone and 17beta-estradiol, via the 5alpha-reductase and aromatase enzymes, respectively. Thus the metabolism of 17beta-TBOH provides a basis for future research evaluating its safety and efficacy as a means of combating muscle and bone wasting conditions, obesity, and/or androgen insensitivity syndromes in humans, similar to that of other SARMs which are currently in development.”
Side note: The study is not on “our” trenbolone but on a 17-beta isomer of trenbolone.

Part of the text is from 2012: //juicedmuscle.com/jmblog/archive/201207


