Hypogondism in young bodybuilders

An increasing number of young and middle‑aged men are seeking treatment for symptoms related to deficient levels of androgens (hypogonadism) including depression, loss of libido, erectile dysfunction, and fatigue. The increase in prevalence of testosterone supplementation in general and anabolic steroid‑induced hypogonadism specifically among younger athletes is creating a population of young men who are uniquely impacted by the testicular end‑organ negative consequences of exogenous steroid use. Exogenous testosterone therapy can alter the natural regulation of the hypothalamic‑pituitary‑gonadal axis leading to impaired spermatogenesis with azoospermia being a serious possible result, thus rendering the individual infertile.
This is not new to most of us, and I often get question about this condition. One I found on a discussionboard (*):

Exogenous testosterone use among men has increased exponentially in the last decade with at least a three‑fold growth in men 40 years old and older. Accumulating evidence demonstrates a significant prevalence of hypogonadism in men over the age of 45, with some estimates reaching 13.8 million hypogonadal men in the United States. At the same time, the age of fatherhood has been increasing. Many physicians are turning to exogenous testosterone supplementation therapy (TST) to help these men with hypogonadism manage symptoms of decreased libido, depression, fatigue, and erectile dysfunction. Unfortunately, use of exogenous testosterone is not limited to medical therapies alone as
use of testosterone, and other anabolic steroids is quite common among young athletes. In the United States, estimates of individuals who use anabolic steroids range from 1 to 3 million people. A substantial proportion of these are young adults. In a study by Buckley et al. 6.6% of high school seniors reported that they used, or had a history of using, anabolic steroids.
Exogenous testosterone use whether for therapeutic or supplementation purposes has serious consequences including diminished sperm production and possible azoospermia, which will impact a man’s ability to establish a pregnancy.
 Epidemiology of hypogonadism in men of reproductive age
Epidemiology of hypogonadism in men of reproductive age
A national, population‑based study using “Clinformatics Data Mart” looked at 10. 739. 815 men aged 40 and older over a period of 10 years (2001–2011). The study found that the percentage of men receiving TST quadrupled in men aged 40–49 and that it had tripled in men aged 50–69. In another retrospective analysis of 410 019 men who initiated testosterone treatment, 12% were men aged 18–39 that is, men of reproductive age. The reasons for this trend are multifactorial and include an increasing population of hypogonadal men, advancing ages of men attempting to establish pregnancy, and the decreases in testosterone levels seen in these men as they age. Harman et al. in their Baltimore Longitudinal Study of Aging looked at the prevalence of hypogonadism as defined by a low testosterone level or free testosterone index, and found that men 20–45 years of age have a 3%–8% incidence of hypogonadism.
The age of fathers per live birth has risen over the last 30 years; more live births are occurring with fathers aged 35–44 than were occurring in 1980. Data emerging from the hypogonadism in males study hints at the fact that a sizeable percentage of men over age 45 have decreased testosterone levels. In the study, which included 13.8 million men over the age of 45, 38.7% had testosterone levels <300 ng dl−1.2 The use of exogenous testosterone is not limited to men with low testosterone levels; it also is found among adolescents, collegiate athletes, and professional and recreative athletes.
Testosterone supplementation impairs fertility In healthy adult males, testosterone production is regulated by the hypothalamic‑pituitary‑gonadal axis. Higher cortical centers in the brain signal the hypothalamus, which in turn modulates the anterior pituitary via pulsatile secretion of gonadotropin‑releasing hormone (GnRH). GnRH leads to the release of LH from the anterior pituitary, which then stimulates the Leydig cells in the testicles to produce testosterone. As testosterone levels increase, negative feedback occurs on both the hypothalamus and the anterior pituitary. Exogenous testosterone use, therefore, results in both impaired endocrine regulation of GnRH and LH release and subsequent decrease of endogenous testosterone.
The use of testosterone supplementation in men of reproductive age could be a form of male contraception, albeit not consistently effective. While such phenomena are not experienced by every patient, exogenous testosterone can lead to the atrophy of the  germinal epithelium in normal men and suppresses spermatogenesis, leading to azoospermia after 10 weeks of use. Testicular atrophy is not uncommon, and it usually is reflective of loss of both spermatogenesis and Leydig cell function. Normal men can be expected to rebound after a period of 6–18 months; however, patients with impaired spermatogenesis at the initiation of androgen supplementation may remain azoospermic (4%–10%), with significant negative consequences if the patient desires children in the future.
germinal epithelium in normal men and suppresses spermatogenesis, leading to azoospermia after 10 weeks of use. Testicular atrophy is not uncommon, and it usually is reflective of loss of both spermatogenesis and Leydig cell function. Normal men can be expected to rebound after a period of 6–18 months; however, patients with impaired spermatogenesis at the initiation of androgen supplementation may remain azoospermic (4%–10%), with significant negative consequences if the patient desires children in the future.
Preventing infertility in hypogonadal males receiving testosterone supplementation therapy
So what options exist for those patients who are hypogonadal and require TST for symptomatic relief? Can we protect these patients from becoming sterile as a result of the negative impact of exogenous testosterone on the hypothalamic‑pituitary‑gonadal axis of these patients?
Human chorionic gonadotropin therapy
 A known critical element in the development of healthy spermatogenesis is high intratesticular testosterone. In men using exogenous testosterone, these levels can be greatly diminished.
A known critical element in the development of healthy spermatogenesis is high intratesticular testosterone. In men using exogenous testosterone, these levels can be greatly diminished.
Intramuscular human chorionic gonadotropin (hCG) therapy is an option shown to protect against, or at least to diminish, the impact that exogenous testosterone has on intratesticular testosterone levels. In a randomized, controlled trial of 29 healthy men randomly assigned to four groups, testosterone enanthate was given 200 mg per week plus either intramuscular saline, 125, 250, or 500 IU hCG every other day. Sperm, intratesticular testosterone levels, and gonadotropins were measured at day 0 and day 21. Intratesticular testosterone levels were suppressed by 94% in the placebo group, 25% in the 125 IU hCG treatment group, and 7% in the 250 IU hCG treatment group, and they were increased 26% from baseline in the 500 IU hCG treatment group. Thus, even with supraphysiologic doses of testosterone replacement, healthy levels of intratesticular testosterone were maintained by low‑dose hCG therapy.
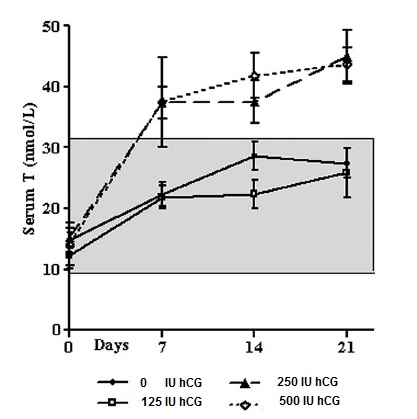 Graphic: Serum T during the treatment phase by group. The shaded box represents the normal reference range of serum T in healthy men for this assay. Serum T increased from baseline in all four groups in response to TE (200 mg, im, weekly and remained elevated during the treatment phase. The two higher hCG dose groups (250 and 500 IU, sc, every other day) had serum T levels above the normal range during the treatment phase !!!
Graphic: Serum T during the treatment phase by group. The shaded box represents the normal reference range of serum T in healthy men for this assay. Serum T increased from baseline in all four groups in response to TE (200 mg, im, weekly and remained elevated during the treatment phase. The two higher hCG dose groups (250 and 500 IU, sc, every other day) had serum T levels above the normal range during the treatment phase !!!
Selective estrogen receptor modulators (SERM)
The ideal testosterone therapy for male patients suffering from hypogonadism not only  would correct signs and symptoms of androgen deficiency, such as decreased libido, erectile dysfunction, and depression, but also would have predictable pharmacokinetics and would not interfere with the patient’s sperm production. In order to make treatment and compliance more realistic and sustainable, testosterone‑enhancing therapy would also come in a form that allowed for a convenient and acceptable route of administration, preferably in pill form.
would correct signs and symptoms of androgen deficiency, such as decreased libido, erectile dysfunction, and depression, but also would have predictable pharmacokinetics and would not interfere with the patient’s sperm production. In order to make treatment and compliance more realistic and sustainable, testosterone‑enhancing therapy would also come in a form that allowed for a convenient and acceptable route of administration, preferably in pill form.
One therapy that may help to alleviate azoospermia and avoid infertility associated with TST is use of selective estrogen receptor modulators (SERMs). SERMs are a class of compounds that act on the estrogen receptor. However, they are unique in that they are not pure receptor agonists and antagonists but have variable effects depending upon the tissue type. In the brain SERMs are antagonists for the estrogen receptors, and act to antagonize the effects of estrogen on the hypothalamus and anterior pituitary.
Clomiphene citrate (Clomid) is a SERM composed of a trans‑isomer and longer acting zu‑isomer. It was first used clinically in the 1960’s to enhance ovulation, but has been used off‑label to treat secondary hypogonadism and male infertility. Through modulation of the estrogen receptors in the hypothalamus and anterior pituitary, of clomiphene citrate not only preserves, but enhances the secretion of FSH and LH from the anterior pituitary. With such treatments, testosterone‑induced azoospermia was successfully reversed with hCG therapy in nearly all men receiving treatment. While further studies need to be carried out, every‑other‑day intramuscular hCG therapy is a viable option in the treatment of men who suffer suppressed spermatogenesis due to testosterone replacement. However, recovery
is not immediate; patient spermatogenesis returned in 4–6 months.
hCG combined with Clomiphene citrate (Clomid)
An algorithm based on historical evidence may be followed in determining the appropriate  course of therapy for men who desire to maintain fertility yet wish to correct their significant symptoms of hypogonadism with TS. In men seeking for hypogonadal symptoms and low testosterone, the first question addressed must be whether fertility is desired. If it is not, the patient may maintain testicular size by adding 1500 IU hCG weekly. If the patient desires to maintain some degree of testicular size, he may cycle off of TST every 6 months, with a 4 week treatment cycle of 3000 IU hCG every other day. If a man does wish to maintain fertility, a baseline semen analysis should be performed and the timeframe for which the patient desires to establish a pregnancy discussed. For those patients desiring to establish a pregnancy within 6 months,
course of therapy for men who desire to maintain fertility yet wish to correct their significant symptoms of hypogonadism with TS. In men seeking for hypogonadal symptoms and low testosterone, the first question addressed must be whether fertility is desired. If it is not, the patient may maintain testicular size by adding 1500 IU hCG weekly. If the patient desires to maintain some degree of testicular size, he may cycle off of TST every 6 months, with a 4 week treatment cycle of 3000 IU hCG every other day. If a man does wish to maintain fertility, a baseline semen analysis should be performed and the timeframe for which the patient desires to establish a pregnancy discussed. For those patients desiring to establish a pregnancy within 6 months,  testosterone therapy should be discontinued, and treatment begun with 3000 IU hCG ± clomiphene citrate (25 mg daily) and a semen analysis performed every 2 months. If the semen analysis remains suboptimal and FSH continues to be suppressed, adding Gonal (FSH) 75 IU to an hCG regimen can be considered. In those patients desiring to establish a pregnancy within 6–12 months, testosterone therapy can be continued with 500 IU hCG every other day ± clomiphene citrate. Those patients desiring to establish a pregnancy after more than 12 months should cycle off testosterone every 6 months with a 4 week cycle of 3000 IU hCG every other day.
testosterone therapy should be discontinued, and treatment begun with 3000 IU hCG ± clomiphene citrate (25 mg daily) and a semen analysis performed every 2 months. If the semen analysis remains suboptimal and FSH continues to be suppressed, adding Gonal (FSH) 75 IU to an hCG regimen can be considered. In those patients desiring to establish a pregnancy within 6–12 months, testosterone therapy can be continued with 500 IU hCG every other day ± clomiphene citrate. Those patients desiring to establish a pregnancy after more than 12 months should cycle off testosterone every 6 months with a 4 week cycle of 3000 IU hCG every other day.
The man with a serum testosterone level of 175 ng dl−1, but desiring to establish a pregnancy within 6 months could be started on 3000 IU of hCG plus clomiphene citrate. This would effectively increase his serum testosterone levels alleviating most hypogonadal symptoms, while maintaining or even enhancing his ability to establish a pregnancy with his wife.
Ramasamy et al 2015 concluded: “The incidence of testosterone supplementation among men during their reproductive years is increasing. Various options exist to re‑initiate sperm production and to mitigate the suppression of spermatogenesis following TST including hCG therapy. These therapies are successful but in a time‑dependent manner. For those men who seek TST because of symptoms of androgen deficiency, concurrent low‑doses of hCG may be a viable option to avoid the azoospermia induced by exogenous testosterone. However, in order to establish hCG treatment as reliable and efficacious a longer study or a larger population is required than that already reported. Enclomiphene citrate, a potent isoform of a SERM, may provide a viable future alternative option for testosterone supplementation that may pose a less negative impact on sperm production while still affording the benefits of increased total testosterone levels.”
Enclomiphene citrate (Androxal) 
Now truthfully as we have discussed in the past clomid (clomiphene citrate) has a significant amount of research showing it increases testosterone to normal levels in hypogonadal men. So why develop Enclomiphene? The answer is simple, it’s what drives every pharma company – profits.
Repros Therapeutics is a pharmaceutical company interested in changing testosterone levels for all men needing some extra help. Repros Therapeutics is developing the drug called Androxal. Androxal is simply the single isomer version of clomiphene citrate (“clomid”), otherwise known as enclomiphene citrate.
Unfortunately clomid is not approved by the FDA to treat hypogonadal males. Otherwise it would be used more frequently. If Repros Therapeutics is able to get FDA approval for enclomiphene (Androxal), there is a very real likelihood this could become the most commonly prescribed method of hormone replacement ever. Now they aren’t fully invested into this product simply for profits, they do believe that the single isomer version of clomid might actually work a bit better.
Now you may be thinking why ditch traditional testosterone injections, and transdermal gels. There are three reasons enclomiphene is superior to test injections and trandermals. First, enclomiphene is an oral pill. Meaning no doctor’s visits for injections, or no botched painful injections by the user themselves. It’s also superior to transdermals because there is no transfer risk to children, females, and animals.
The second reason why this is superior to testosterone replacement is that it does not suppress follicle stimulating hormone (FSH), and leutinizing hormone (LH), and subsequently can maintain (not suppress) fertility. Being able to conceive a child is important to many men.
The third and final reason, which is simply cosmetic, is that enclomiphene maintains testicular size. Unfortunately one side effect of testosterone replacement is shrunken nut
 There are many different ways to reach the goal if you want to get rid of depression, no drive to go to the gym, loss of libido, erectile dysfunction, shrunken nuts and fatigue. Of course hCG is used mostly but sometimes other compounds like hMG ( human menopausal gonadotropin) work even better. Like with everything else everyone is different. And like I wrote in my previous post TST or HRT is now often done with compounds like Aromasin and when Enclomophene is approved … It’s still an open end.
There are many different ways to reach the goal if you want to get rid of depression, no drive to go to the gym, loss of libido, erectile dysfunction, shrunken nuts and fatigue. Of course hCG is used mostly but sometimes other compounds like hMG ( human menopausal gonadotropin) work even better. Like with everything else everyone is different. And like I wrote in my previous post TST or HRT is now often done with compounds like Aromasin and when Enclomophene is approved … It’s still an open end.



