Growth Hormone part 1
The Many Faces of Growth Hormone
BY RONNY TOBER 2005
This is an article I wrote for a bodybuilding magazine in 2005. The information is therefore dated. But it contains usefull information and newer information about this subject can be found in newer articles.
 Human growth hormone (somatropin) is widely used outside of normal medical situations. For example, in bodybuilding it is taken to increase muscle mass and decrease body fat. Being so far removed from the hands of legitimate pharmaceutical distributors, doctors, and pharmacists, preparations of doubtful origin and low quality frequently find their way to the (black) market.
Human growth hormone (somatropin) is widely used outside of normal medical situations. For example, in bodybuilding it is taken to increase muscle mass and decrease body fat. Being so far removed from the hands of legitimate pharmaceutical distributors, doctors, and pharmacists, preparations of doubtful origin and low quality frequently find their way to the (black) market.
In the last few years,counterfeited, contaminated, and very poor quality somatropin have been located and analysed, both in the European Union and in the United States. There were many counterfeited preparations found, some of which instead of the expected recombinant growth hormone rhGH, contained the pregnancy hormone HCG. Other illegally prepared preparations contained growth hormone from human cadavers (pituitary glands), veterinary somatropin variants, vitamins, insulin,etc. Looking at the more than 50 proven cases of the transmission of the Creutzfeldt Jakob Disease by contaminated growth hormone preparations, mainly during the 1980’s [1-4],we feel people should be urgently warned before using questionable growth hormone preparations [5-9]. This article will take a close look at the various manufacturing techniques used to make growth hormone drugs, placing a strong emphasis on how to identify quality preparations from those of a more questionable nature.
The Biosynthesis of Somatropin
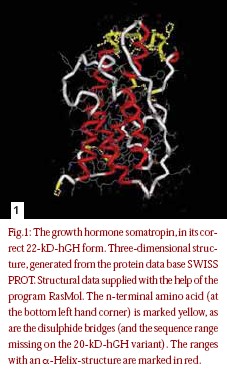
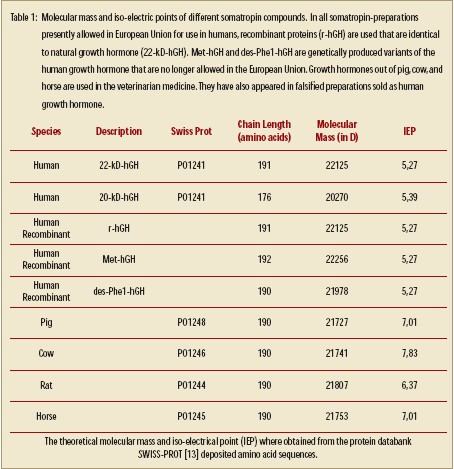
Before we get into the manufacturing of GH drugs, it is important to understand how the body makes this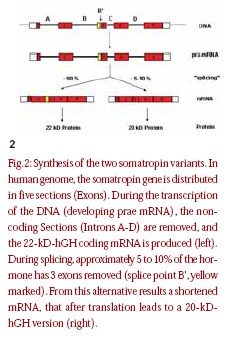 hormone. The human growth hormone somatropin (hGH) is a protein hormone with a chain length of 191 amino acids and a molecular mass of approx. 22 kD. The tertiary period structure of the protein becomes stabilized by two disulphide bridges (fig.1).The endogenous synthesis and release of growth hormone takes place in the pituitary gland, under control of hypothalamus peptide hormones. Endogenous somatropin is first synthesized as a precursor hormone 217 amino acids in length. During secretion, a signal peptide with 26 amino acids is spliced from the hormone, yielding the active GH molecule [10]. The target gene for human growth hormone covers five coding sections of the primary transcripts become removed during its subsequent treatment [11]. In the majority of the cases, the result is one mRNA for the complete growth hormone with a chain length of 191 amino acids and a mass of approx.22 kD (22-kD hGH). A certain portion mRNA is, however, subject to an alternate splicing process. It develops, among other things, one 45 nucleotide shortened variant mRNA, which after translation into those appropriate protein sequences leads to a variant of growth hormone with the amino acids in position 32-46 missing (fig. 2).The resulting 20-kDhGH is not formed during genetic manufacturing, which is based on the already correctly spliced gene. It can be used, therefore, as a marker to identify growth hormone preparations taken from human pituitaries [11–12].Table 1 gives an overview over the different growth hormones.
hormone. The human growth hormone somatropin (hGH) is a protein hormone with a chain length of 191 amino acids and a molecular mass of approx. 22 kD. The tertiary period structure of the protein becomes stabilized by two disulphide bridges (fig.1).The endogenous synthesis and release of growth hormone takes place in the pituitary gland, under control of hypothalamus peptide hormones. Endogenous somatropin is first synthesized as a precursor hormone 217 amino acids in length. During secretion, a signal peptide with 26 amino acids is spliced from the hormone, yielding the active GH molecule [10]. The target gene for human growth hormone covers five coding sections of the primary transcripts become removed during its subsequent treatment [11]. In the majority of the cases, the result is one mRNA for the complete growth hormone with a chain length of 191 amino acids and a mass of approx.22 kD (22-kD hGH). A certain portion mRNA is, however, subject to an alternate splicing process. It develops, among other things, one 45 nucleotide shortened variant mRNA, which after translation into those appropriate protein sequences leads to a variant of growth hormone with the amino acids in position 32-46 missing (fig. 2).The resulting 20-kDhGH is not formed during genetic manufacturing, which is based on the already correctly spliced gene. It can be used, therefore, as a marker to identify growth hormone preparations taken from human pituitaries [11–12].Table 1 gives an overview over the different growth hormones.
Cadaveric Growth Hormone
 Before the introduction of genetic methods of manufacturing recombinant proteins, human growth hormone was obtained by extracting it from the pituitaries of corpses. Because of the risk of the transmission of infectious diseases such as the rare neurological disorder Creutzfeldt-Jakob-Desease [1 –4],and other infectious diseases such as hepatitis B and C, growth hormone preparations extracted from human pituitaries were taken off the market in the European Union in the middle of the 1980’s [17]. After that point, only genetically produced (synthetic) growth hormone preparations have been legal in the E.U. Such products are manufactured by recombinant technology.
Before the introduction of genetic methods of manufacturing recombinant proteins, human growth hormone was obtained by extracting it from the pituitaries of corpses. Because of the risk of the transmission of infectious diseases such as the rare neurological disorder Creutzfeldt-Jakob-Desease [1 –4],and other infectious diseases such as hepatitis B and C, growth hormone preparations extracted from human pituitaries were taken off the market in the European Union in the middle of the 1980’s [17]. After that point, only genetically produced (synthetic) growth hormone preparations have been legal in the E.U. Such products are manufactured by recombinant technology.
Growth hormone preparations manufactured with human cadaveric pituitaries are still available on the black market in Europe. If an athlete uses such preparations, they need to understand that a risk of the transmission of disease exists.Because the risks of a medication aren’t always disclosed in the underground drug scene, a slighter lower price might be allowed to be the decisive factor in purchasing a risky biological GH product. Nevertheless, we urgently warn against using such preparations.
Inclusion Body Technology/Somatrem
Clinical trials using synthetic human growth hormone began in the early 1980’s.By 1985, recombinant human growth hormone became available commercially. One of the first certified products in the USA in those years was Protropin‚ by Genentech Inc. This product differs from more popular GH remedies of today like Nutropin, Saizen and Humatrope, in that it contains somatrem instead of somatropin. Somatrem is a synthetic GH protein that contains an additional methionine amino acid, which has been added to the 24-amino acid initiation sequence. Natural (endogenous) human growth hormone is a polypeptide containing 191 amino acids. The biosynthetic process used to make somatrem, also called the Inclusion Body Process, involves the chemical synthesis of the DNA fragment encoding the first 24 amino acids. The remaining amino acid residues are obtained by making complimentary DNA copies of messenger RNA prepared from human pituitary cells. The entire DNA sequence is introduced into a bacterium, escherichia coli (E.coli),which is then able to synthesize the GH protein. Somatrem, known chemically as MethGH or N-methionyl-hGH, was first believed to be equal to endogenous growth hormone (with 191 amino acids) in all regards. That was perhaps only because they compared it to biological (corpse) growth hormone, which was not pure, and contained pollutants that produced side effects in many users. When scientists finally succeeded in producing the correct human growth hormone protein with 191 amino acids (via secretion technology), they immediately noticed the difference. It turned out that the extra amino acid was making the body think that it was being invaded by a foreign body, and in turn was causing it to produce antibodies against this hormone in many users. This led to allergic reactions, and sometimes the antibodies are so prominent that they even neutralised the effect of the administered hormone. Antibodies were noted in approximitely 50% of the young users in one clinical experiment [41], and a second British experiment rated the response even higher, in 80% of users [42].The antibodies had no serious side effects to note, however. Last year there was this article about a young user taking Met-hGH and stopped growing. The doctors stopped administration of Met-hGh, and after a seven month break, started treatment with rhGH (191 amino acids), and growth resumed [43]. If you do use somatrem, this form of GH must be kept at 2-8° C to maintain stability.
Protein Secretion Technology & Mouse-cell Manufacturing
Protein secretion technology is an improved alternative to production in e-coli, in which the rhGh is secreted in a protein-free production media. Manufacturing a recombinant protein like human growth hormone by secretion is done by adding a leader of secretory sequence (usually a sequence of a cytokine) onto the n-terminal (nitrogenterminal) of the growth hormone amino acid chain. That allows the recombinant protein to be secreted, facilitating a simpler purification process, and avoiding resolubisation of inclusion bodies and protein refolding. The secretion technology of protein leads to complete removal of n-terminal amino acid residue on synthesised proteins, a problem with production with E. coli (Inclusion Body).One exception to this is the preparation Saizen® from Ares-Serono, which is produced with the use of mousecell material C127.Because of the controlled genetic manufacturing, there is no risk of patients being infected with CJD with the Protein Secretion, Inclusion Body,or Mousecell manufacturing technologies. Currently, the only allowed growth hormone preparations for therapeutic use on the regular E.U. medicine market come from the firms Lilly (Humatrope®), Pharmacia (Genotropin®), NovoNordisk (Norditropin®), Ferring (Zomacton®), and Ares-Serono (Saizen®). These preparations contain genetically produced hGH, identical in structure to the correct human growth hormone protein with a mass of ca. 22 kD (r-hGH). They are allowed for the treatment of children with short stature, before termination of their normal growth phase, and/or for replacement therapy in adults with growth hormone deficiency. Quality is guaranteed through the procedures and controlled manufacturing of GMP-conditions.Why is it important how your rhGh is manufatured and purified? Because rhGH is a very large and fragile protein molecule, which is carefully folded and twisted on itself. Activity in the body depends on the highly fragile cross-linkages between the protein branches, which hold it in an exact three dimensional configuration. It works on cell receptors only when this specifically oriented key configuration fits its specific receptors-site lock. Any change in shape can block its biological activity. That is why an apropriate manufacturing, purifying,and refolding of somatropin are so important. Another advantage to point out, is that growth hormone manufactured via secretion technology is very stable, and can be shipped at temperatures of 37° Celsius or 98° Fahrenheit. Older Inclusion Body manufacturing technology, like that used to make Protropin, requires that the hormone be kept at 2-8°C,which means that shipment must be done with dry ice, and the product must be kept refrigerated at all other times. And we all know with black market drugs, that this is not always the case.
Immugenic Reactions and Antibodies (GH-AB)
Red welts, painful injection spots, and increased site temperature are indicative of an immune response to GH injections. This could be caused by residual bacterial protein from the inclusion bodies, but it is also very possible that it is caused by somatrem. The additional amino acid, methionine, is enough of a difference that the immune system recognizes it as a foreign protein in 50-80% of the users in these studies [41-42]. In the worst scenario, this can lead to an immune response against all forms of hGH including the endogeneous GH, a kind of autoimmune response. If the immune system recognizes a variant like Met-somatropin or des-phe1-somatropin as foreign, and starts an immune response to it, the possibility exists that the antibodies can turn against endogenous growth hormone as well. This is because, aside from the extra or missing amino acid, the rest of the growth hormone sequence is identical to endogeneous growth hormone. Concerning the source of the GH-antibodies, this study [44] suggest that the immunogenicity was not due to the n-terminal methionine or e.coli protein impurities, but rather was probably caused by small amounts of growth hormone with subtle structural alterations whose life remains unknown. This study [45] confirms the high immunogenicity of Met-somatropin, especially in patients not treated earlier with rhGH.Once present, the GH-AB remains detectable throughout the period of treatment with Met-somatropin, however after stopping met-rhGH,or changing to 22kD-rhGH,the GH-AB disappeared rapidly in most patients.
Brand Analysis
 Test samples of Somatogen-L® were analysed in the years 1996 to 1998, and deviations from authentic growth hormone were noticeable for the first time in the peptide mapping. After enzymatic dismantling of the protein through trypsine and chromatographical separation of the fragments, it was noticed that a different peak pattern in comparison with the reference substance was noticed in the Somatogen-L samples. The identity test through reversed-phase HPLC (identity test of the medicine bookmonograph) revealed several peaks with different retention times in comparison with the somatropin reference substance. In regards to the expected correct somatropin retention times, no peak appeared. Molecular mass was determined through LCMS with Elektrospray-Ionisation (ESI), and yielded a value lower than the reference substance. The difference in mass measured was 147 D,which corresponded exactly with the mass of a phenylalanine in the peptide chain. Because both the N-terminal and the C-terminal of the somatropin chain begins with phenylalanine [13], and within the peptide chain are also several phenylalanine building blocks, a sequencisation was required in order to determine the exact position of the deletion. Fortunately, the sequencisation of the N-terminal delivered the necessary additional information. It turned out that the active agent of the examined preparations was des-Phe1-Somatropin, which in comparison to the 22-kD-hGH with 191 amino acids is missing the first (N-terminal) amino acid [31]. Next, reversed-phase chromatography SDSPAGE and IEF delivered unambiguous references to proteolytical dismantling and other protein dismantling products. Through desamidierung and s-oxidation of the amino acids side chain, such products can emerge, usually with improper storage.In the test, the request of the medicine book monograph on related proteins and in the IEF-testcompounds where not completed.
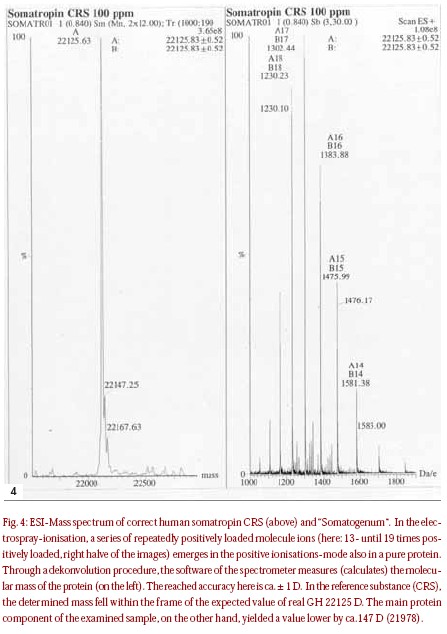
Test samples of Somatogen-L® were analysed in the years 1996 to 1998, and deviations from authentic growth hormone were noticeable for the first time in the peptide mapping. After enzymatic dismantling of the protein through trypsine and chromatographical separation of the fragments, it was noticed that a different peak pattern in comparison with the reference substance was noticed in the Somatogen-L samples. The identity test through reversed-phase HPLC (identity test of the medicine bookmonograph) revealed several peaks with different retention times in comparison with the somatropin reference substance. In regards to the expected correct somatropin retention times, no peak appeared. Molecular mass was determined through LCMS with Elektrospray-Ionisation (ESI), and yielded a value lower than the reference substance. The difference in mass measured was 147 D,which corresponded exactly with the mass of a phenylalanine in the peptide chain. Because both the N-terminal and the C-terminal of the somatropin chain begins with phenylalanine [13], and within the peptide chain are also several phenylalanine building blocks, a sequencisation was required in order to determine the exact position of the deletion. Fortunately, the sequencisation of the N-terminal delivered the necessary additional information. It turned out that the active agent of the examined preparations was des-Phe1-Somatropin, which in comparison to the 22-kD-hGH with 191 amino acids is missing the first (N-terminal) amino acid [31]. Next, reversed-phase chromatography SDSPAGE and IEF delivered unambiguous references to proteolytical dismantling and other protein dismantling products. Through desamidierung and s-oxidation of the amino acids side chain, such products can emerge, usually with improper storage.In the test, the request of the medicine book monograph on related proteins and in the IEF-testcompounds where not completed. 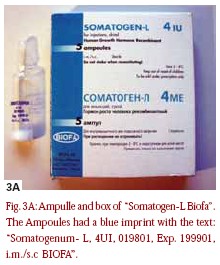 However, the fact that the 20-kD-hGH protein was missing in the tests for pituitary cadavaric extract allowed the end conclusion that the examined samples (years 1996 to 1998) concerned no material made from human pituitaries, but rather with high probability,was genetically produced. More recently, samples of Somatogenum were sent into the lab for analysis via electro-spray-mass-spectrometry (Fig 4). The samples were dated in 1999, and yielded a result that also pointed to the genetically produced active agent des-Phe1-Somatropin. In the reference substance (CRS), the middle molecular mass was determined by ESI mass-spectrometry to be 22125,8 ± 0.5 D,which matches very well the amino acids sequence of the two intermolecular disulfide bridges calculated with an expectation value of 22125 D. The “Somatogenum” sample had again produced a signal with a clearly lower mass 21978,1 ± 0.3 D,(measured difference: ca.147 D), again like would be expected for a des-Phe1-Somatropin.With a further testing through peptide mapping or reversed- phase HPLC, the material became clearer. In contrast to the samples examined in the previous years with the label “Somatogenum”, the results of the purity tests were verified by means of IEF and SDS-PAGE and compared with somatropin CRS, and both in the IEF and in the SDS-PAGE the sample showed only main volumes in the expected pH- and/or molecular measured area. No reference arose to protein dismantling products. Obviously it showed that the examined sample contained a chemical that was clean and undecomposed. Corpormon® is a GH product produced by Nikken Japan. The analysis of two samples found on the black market failed the identity test when compared with Somatropin via reversed-phase HPLC. Instead of the peaks with expected retention time of ca. 30 minutes, several peaks were observed in very short retention times, after 5 to 8 minutes. An Internet-search on Corpormon in the bodybuilding scene showed that the preparation is frequently available on the black market, and is suspected to be a counterfeit which contains hCG instead of recombinant somatropin. This suspicion was confirmed in the samples examined here. A conventional pregnancy test carried out in corresponding thinning to the highly selective immunologic proof by hCG was positive in both samples. The result was confirmed through SDS-PAGE. Both Corpormon samples yielded characteristic volumes for the alphas and lower unit of hCG. (a) The selected chromatographical conditions for the reference substance showed that the peak was to expect after ca.30 min. (b) With Corpormon 4UI this peak was missing. Instead, several peaks stepped in very short retention times of 5 to 8 min on.(c) A retroactively examined hCG-containing preparation also showed a peak in ca 5 min.
However, the fact that the 20-kD-hGH protein was missing in the tests for pituitary cadavaric extract allowed the end conclusion that the examined samples (years 1996 to 1998) concerned no material made from human pituitaries, but rather with high probability,was genetically produced. More recently, samples of Somatogenum were sent into the lab for analysis via electro-spray-mass-spectrometry (Fig 4). The samples were dated in 1999, and yielded a result that also pointed to the genetically produced active agent des-Phe1-Somatropin. In the reference substance (CRS), the middle molecular mass was determined by ESI mass-spectrometry to be 22125,8 ± 0.5 D,which matches very well the amino acids sequence of the two intermolecular disulfide bridges calculated with an expectation value of 22125 D. The “Somatogenum” sample had again produced a signal with a clearly lower mass 21978,1 ± 0.3 D,(measured difference: ca.147 D), again like would be expected for a des-Phe1-Somatropin.With a further testing through peptide mapping or reversed- phase HPLC, the material became clearer. In contrast to the samples examined in the previous years with the label “Somatogenum”, the results of the purity tests were verified by means of IEF and SDS-PAGE and compared with somatropin CRS, and both in the IEF and in the SDS-PAGE the sample showed only main volumes in the expected pH- and/or molecular measured area. No reference arose to protein dismantling products. Obviously it showed that the examined sample contained a chemical that was clean and undecomposed. Corpormon® is a GH product produced by Nikken Japan. The analysis of two samples found on the black market failed the identity test when compared with Somatropin via reversed-phase HPLC. Instead of the peaks with expected retention time of ca. 30 minutes, several peaks were observed in very short retention times, after 5 to 8 minutes. An Internet-search on Corpormon in the bodybuilding scene showed that the preparation is frequently available on the black market, and is suspected to be a counterfeit which contains hCG instead of recombinant somatropin. This suspicion was confirmed in the samples examined here. A conventional pregnancy test carried out in corresponding thinning to the highly selective immunologic proof by hCG was positive in both samples. The result was confirmed through SDS-PAGE. Both Corpormon samples yielded characteristic volumes for the alphas and lower unit of hCG. (a) The selected chromatographical conditions for the reference substance showed that the peak was to expect after ca.30 min. (b) With Corpormon 4UI this peak was missing. Instead, several peaks stepped in very short retention times of 5 to 8 min on.(c) A retroactively examined hCG-containing preparation also showed a peak in ca 5 min.
Risks because of missing EU-allowance or falsification
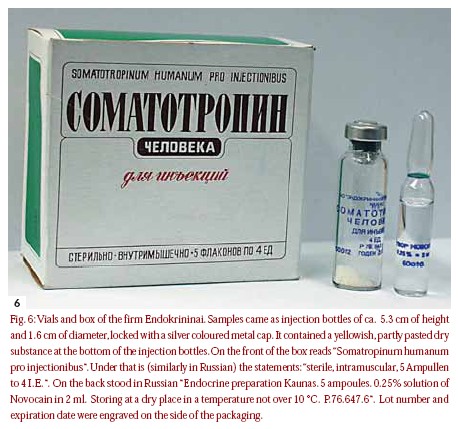
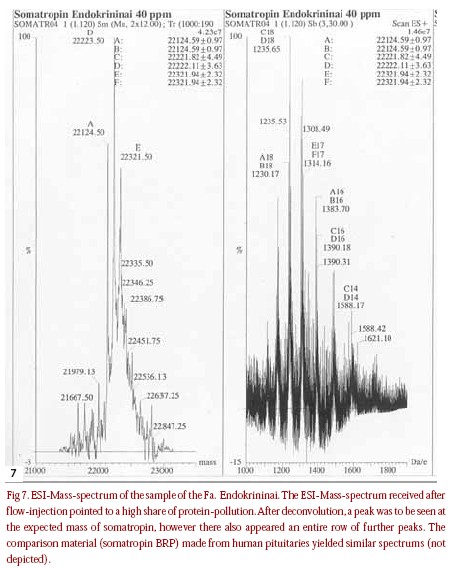
All previously examined samples with the label “Somatogen/Somatogenum”contained the active agent  des-Phe1 somatropin. Preparations with this active agent are to be classified as genetically made,but of the first generation of such products, comparable to Met-Somatropin (somatrem). As genetically produced medicines, they could be allowed within the EU, centrally obligatorily over the European allowance authority EMEA in London. But because corresponding allowance does not exist, preparations with the active agent of Phe1-Somatropin are presently not used in the EU. In Lithuania and some other East European countries, a medicine of the label “Somatogenum- L 4 UI”from the company Biofa, which contains des-Phe1-Somatropin, is registered for the treatment of growth interferences [34]. In the products found on the black market with the label “Somatogenum”, it could therefore be either original products out of East European countries or counterfeits. A test on security, effectiveness, and identity did not take place within the EU. Because the manufacture conditions are not disclosed, an infection risk by possible contamination with pathogenic germs cannot be excluded. In Japan (however not in the EU), a finished medicine with the label “Corpormon 4UI” from the firm Nikken with genetically produced des- Phe1 somatropin as an active agent is allowed. In the samples examined here with the label “Corpormon”,we obviously examined forgeries that contained the pregnancy hormone hCG. A sample was sent in for analysis,which contained on the vial the text “COMATOTPOHNH”, Fa.Endokrininai”. After the isoelectrical focussing, several intense peaks in the pH-area of 4.6 to 5.2 showed up in the examined sample, next to main peaks in pH-5.2. In the older comparison substance (BRP) made from cadaveric pituitaries, similar secondary peaks were also observed. The secondary peaks are to be led back probably to the hydrolysis of asparagine-and glutamine- side chain of the protein to the corresponding desamido-forms. Through the transformation of the acidamidgroups in carbonacidgroups, the isoelectrical point of the protein shifts itself into the sour area. Preferred attack points for the hydrolytical dismantling are the exposed acidamidgroups of asparagine in position 149 and 152 of the polypeptide-chain [32, 33]. The demand of the medicine book monograph according to secondary peaks with an intensity of no more than 6.25% of the intensity of the head volumes may appear, was therefore not fulfilled in the analysed preparation. In the test on related substances by means of reversed-phase HPLC, a peak was seen in the retention time of somatropin, but several intensive secondary peaks with shorter retention times also occured. The purity request of the medicine book-monograph was not fulfilled (sum of the peakareas of the secondary peaks <13%) for this sample. By means of ESIMass- spectrometry, the active agent somatropin could be proved unambiguously by the mass-spectrometrical detection of the molecule ion in the expected mass at 22,125 D in the sample. The additional observed intensive signals in 22,224 D and in 22,322 D and the altogether quite unclean measured spectrum referred to pollution through accompanying proteins and decomposition products. In the analysis through SDS-Polyacrylamide electrophorese, the sample clearly showed numerous additional volumes next to the head volumes in 22 kD and a. in 20 kD and 44 kD. The model of the protein pollution was very similar to that of the comparison substance (BRP),made from cadaveric pituitary material. The presence of that 20- kD-volumes in the sample proved that it concerned indeed a growth hormone preparation made out of human pituitaries, not synthetic rHGH!
des-Phe1 somatropin. Preparations with this active agent are to be classified as genetically made,but of the first generation of such products, comparable to Met-Somatropin (somatrem). As genetically produced medicines, they could be allowed within the EU, centrally obligatorily over the European allowance authority EMEA in London. But because corresponding allowance does not exist, preparations with the active agent of Phe1-Somatropin are presently not used in the EU. In Lithuania and some other East European countries, a medicine of the label “Somatogenum- L 4 UI”from the company Biofa, which contains des-Phe1-Somatropin, is registered for the treatment of growth interferences [34]. In the products found on the black market with the label “Somatogenum”, it could therefore be either original products out of East European countries or counterfeits. A test on security, effectiveness, and identity did not take place within the EU. Because the manufacture conditions are not disclosed, an infection risk by possible contamination with pathogenic germs cannot be excluded. In Japan (however not in the EU), a finished medicine with the label “Corpormon 4UI” from the firm Nikken with genetically produced des- Phe1 somatropin as an active agent is allowed. In the samples examined here with the label “Corpormon”,we obviously examined forgeries that contained the pregnancy hormone hCG. A sample was sent in for analysis,which contained on the vial the text “COMATOTPOHNH”, Fa.Endokrininai”. After the isoelectrical focussing, several intense peaks in the pH-area of 4.6 to 5.2 showed up in the examined sample, next to main peaks in pH-5.2. In the older comparison substance (BRP) made from cadaveric pituitaries, similar secondary peaks were also observed. The secondary peaks are to be led back probably to the hydrolysis of asparagine-and glutamine- side chain of the protein to the corresponding desamido-forms. Through the transformation of the acidamidgroups in carbonacidgroups, the isoelectrical point of the protein shifts itself into the sour area. Preferred attack points for the hydrolytical dismantling are the exposed acidamidgroups of asparagine in position 149 and 152 of the polypeptide-chain [32, 33]. The demand of the medicine book monograph according to secondary peaks with an intensity of no more than 6.25% of the intensity of the head volumes may appear, was therefore not fulfilled in the analysed preparation. In the test on related substances by means of reversed-phase HPLC, a peak was seen in the retention time of somatropin, but several intensive secondary peaks with shorter retention times also occured. The purity request of the medicine book-monograph was not fulfilled (sum of the peakareas of the secondary peaks <13%) for this sample. By means of ESIMass- spectrometry, the active agent somatropin could be proved unambiguously by the mass-spectrometrical detection of the molecule ion in the expected mass at 22,125 D in the sample. The additional observed intensive signals in 22,224 D and in 22,322 D and the altogether quite unclean measured spectrum referred to pollution through accompanying proteins and decomposition products. In the analysis through SDS-Polyacrylamide electrophorese, the sample clearly showed numerous additional volumes next to the head volumes in 22 kD and a. in 20 kD and 44 kD. The model of the protein pollution was very similar to that of the comparison substance (BRP),made from cadaveric pituitary material. The presence of that 20- kD-volumes in the sample proved that it concerned indeed a growth hormone preparation made out of human pituitaries, not synthetic rHGH!
Chinese Growth Hormone
An ampoule of Ansomone®‚ was offered to the official Dutch anti-doping agency for analyses in January 2003. It turned out that the ampoule contained Met-hGH or Nmethionyl- hGH (somatrem; the 192 amino acid variant of hGH), instead of the correct 191 amino acid sequence rhGH it was labeled to contain.The pharmaceutical producer Anhui Anke Biotechnology tells a whole different story on its website. According to the Chinese producer, mostly called AnkeBio,“Ansome is a compound produced by modified bacteria, that is 100% equal to growth hormone made in humans: somatropin”. The lab found out that the dose of somatrem was okay,and that it didn’t possess much protein that could pollute the preparation. No bathtub pharmacy for that matter [47], although it isn’t exactly what you are paying for. We asked AnkeBio for a reaction on this article, and they stated it was only a rumor, and that their rhGH was as good as any other brand. GenLei told us that similar analyses were done in China, and they had the same results. Genescience Jintropin® is manufactured by secretion technology. Genescience produces it specifically via Dr Jin’s secretion technology, hence the name Jintropin. This secretion technology is patented in China by Genescience, which is why other Chinese manufacturers cannot use this technology to produce rhGH, despite its many advantages. In August 2004 GeneScience changed their design of Jintropin.The 10 iu vials have a yellow top with “Jintropin 10 iu” indented in the plastic.The same is true for the 4 iu vials, but they have a green top. On that date the old holograms where replaced with security stickers from Orient anti-counterfeiting international. Chinese manufacturers often use (official) distributors, export agents,or shipping agents to get their products out of the country. GenSci informed us that due to the many fakes and counterfeits they would only be exporting themselves directly to customers from now on According to IMS-Health ,GeneScience has a +75% market-share on GH products in the Chinese market. Their brand has become increasingly popular, especially with bodybuilders that use high amounts, and find favor in its excellent quality and low price. It’s no wonder that more and more suppliers and counterfeiters want to try to take a piece of the cake! The first counterfeits of any well-known brand are always remembered in a notorious nature. With Gene- Science, this happened with vials that carried green tops and paper labels, bearing Chinese lettering and the batch number 20020515.
Counterfeiting is a subject that GeneScience tried to avoid in our conversation. They admit that this crime is an increasing factor in the market. One “counterfeit” looks exactly like the Jintropin box, and Chinese agents sell it as a cheaper product made secretly by the real GeneScience company (called Genitropin from ProGeni).GeneScience mailed us denying this, and stating that such a policy would be detrimental with their product image and quality.
International Pharmaceuticals (IP China) claims on their website that they sell somatropin,” synthetic HGH aka rhGH, we have specially ordered production of 191 amino acid version, same as Humatrope and Saizen, which is an exact duplicate of bodies own, guaranteed against any lab test, vials labelled with batch# and Exp date, it’s branded and production regulated by Chinese SDA (equivelant of US-FDA), however the factory asked us not to reveal the brand name..” Sure mate, and the Earth is flat.
Fitropin® is another Chinese brand of GH that advertises on the bodybuilding forums. It is made by the pharmaceutical firm Kexing. Fitropin is another Chinese GH product that is made using inclusion body technology. The manufacturer openly discloses the fact that they sell somatrem instead of somatropin, so this is not a point of grievance with the maker. Because all Chinese rhGH manufactures (Kexing- United etc) except GeneScience (Jintropin) still use the inclusion body technology, it is very well possible that all of them are just purchasing from a single large manufacturer.
Other Fake GH Products/Analysis Results
 A vial Somatohorm® growth hormone compound was analysed through Ergogenics in January 2003. It did not contain growth hormone,but a substance that caused violent pain after the injection. The reason became clear after analysis by an accredited laboratory. The scientists first searched in the sample for peptide pieces between 5 and the 220 kilo daltons. Because they did not find any, that excluded that the ampoule contained the correct 4 iu of GH. This analysis took place using SDS-PAGE and LC-MS. Because the owner of the compound had got violent pain after injection, the scientists searched in the ampoule further for toxicological substances. With Hplc-DAD method they found out that the ampoule contained a thiamine derivate, which at closer research with LC-MS proved to be thiaminediphosphate, a derivative of the vitamin B1 that is not suitable for injection. The valued quantity was estimated at 25 milligram per ampoule. Because the scientists had performed already some analyses with the ampoule they could not determine the exact quality. That vitamin alternative was produced as a byproduct/contaminant of inclusion body technology, and it was causing injection pain. When dissolved in water thiaminediphosphate reduces pH. Three milligram of this substance in 1 millilitre of water decreases the pH to 2.2. Injection carrier, whether you want to inject subcutanously (under the skin) or intramuscularly (in the muscle), must have a Ph value between 4 and 9.At other values the injections are painful and the tissue around the needle spot can die (necroses). The examined ampoule came from Poland. Lately Somatohorm® comes mostly from the Czech Republic [47]. This is not the first time that counterfeited Somatohorm® has been spotted. The world health organization WHO reported also about falsified Somatohorm® between 1999 and 2000. Those fakes appeared to contain no active substances upon analysis. During the same period, the WHO reported an ampoule of Serostim that not valid even to the eye, and a fake ampoule of Breeza Tech®‚ was human growth hormone that did not contain somatropin, but actually the veterinary Methionyl equine somatotropin [46]. On April 2004 fake Serostim® with the batch numbers MNK612A and MNH605A and an expiration date of 08/02 was found.
A vial Somatohorm® growth hormone compound was analysed through Ergogenics in January 2003. It did not contain growth hormone,but a substance that caused violent pain after the injection. The reason became clear after analysis by an accredited laboratory. The scientists first searched in the sample for peptide pieces between 5 and the 220 kilo daltons. Because they did not find any, that excluded that the ampoule contained the correct 4 iu of GH. This analysis took place using SDS-PAGE and LC-MS. Because the owner of the compound had got violent pain after injection, the scientists searched in the ampoule further for toxicological substances. With Hplc-DAD method they found out that the ampoule contained a thiamine derivate, which at closer research with LC-MS proved to be thiaminediphosphate, a derivative of the vitamin B1 that is not suitable for injection. The valued quantity was estimated at 25 milligram per ampoule. Because the scientists had performed already some analyses with the ampoule they could not determine the exact quality. That vitamin alternative was produced as a byproduct/contaminant of inclusion body technology, and it was causing injection pain. When dissolved in water thiaminediphosphate reduces pH. Three milligram of this substance in 1 millilitre of water decreases the pH to 2.2. Injection carrier, whether you want to inject subcutanously (under the skin) or intramuscularly (in the muscle), must have a Ph value between 4 and 9.At other values the injections are painful and the tissue around the needle spot can die (necroses). The examined ampoule came from Poland. Lately Somatohorm® comes mostly from the Czech Republic [47]. This is not the first time that counterfeited Somatohorm® has been spotted. The world health organization WHO reported also about falsified Somatohorm® between 1999 and 2000. Those fakes appeared to contain no active substances upon analysis. During the same period, the WHO reported an ampoule of Serostim that not valid even to the eye, and a fake ampoule of Breeza Tech®‚ was human growth hormone that did not contain somatropin, but actually the veterinary Methionyl equine somatotropin [46]. On April 2004 fake Serostim® with the batch numbers MNK612A and MNH605A and an expiration date of 08/02 was found.
 While the actual drug has the same batch number, its correct expiration date should be 08/01. It came as a kit with eight ampoules, and cost 150 dollars a piece. The producer of the counterfeited Serostim was no unknown person to the police force, and had already sold anabolic androgenic steroids since 1995. At his house in Mason, Iowa the police force found four hundred thousand dollars in cash, and said that he had supplied growth hormone since 2001. The police also retrieved a supply of two thousand boxes, with a street value of four hundred thousand dollars. Preliminary analysis of this counterfeit lot by the FDA indicates that it contained 1 mg rhGH instead of the labeled 6 mg.This product was so good looking that it even made its way to the pharmacy shelves! [49].
While the actual drug has the same batch number, its correct expiration date should be 08/01. It came as a kit with eight ampoules, and cost 150 dollars a piece. The producer of the counterfeited Serostim was no unknown person to the police force, and had already sold anabolic androgenic steroids since 1995. At his house in Mason, Iowa the police force found four hundred thousand dollars in cash, and said that he had supplied growth hormone since 2001. The police also retrieved a supply of two thousand boxes, with a street value of four hundred thousand dollars. Preliminary analysis of this counterfeit lot by the FDA indicates that it contained 1 mg rhGH instead of the labeled 6 mg.This product was so good looking that it even made its way to the pharmacy shelves! [49].
In August 2001 the FDA forensic laboratory examined seven vials of counterfeit Nutropin AQ®, and found that lot number L9101A4 and L9043A4 contained human insulin. Sample vials of counterfeit lot number L9504A2 and L9504A3 did not contain any active ingredient.
The Finnish National office of Investigation had warned during Christmas 2002 about falsified Genotropin®.Reason was a raid on a doping house that took place mid-December. At those raids, agents in Jyväskylä found a couple false ampoules growth hormone [48]. At closer research, it appeared that the ampoules in fact were original Caverject ampoules. The falsifiers had them covered with new labels. The national office suspects that the black market circulates more falsified ampoules of Genotropin that in fact contains Caverject. Genotropin® is twenty times more expensive than Caverject, so we can understand the financial motives. The active substance in Caverject is prostaglandin E1. Impotent men inject it in their penis to bring about a erection.After injection you develop a burning sensation feeling around the needle spot.
The take home message: GH isn’t just GH! Shop carefully! Until next time. – RT
References:
[1] Brown P., Preece M.A. and Will R.G.: “Friendly fire” in medicine: hormones, homografts, and Creutzfeldt-Jakob disease.Lancet 340, 24 – 27 (1992).
[2] Billette de Villemeur T. et al.:Creutzfeldt- Jakob disease from contaminated growth hormone extracts in France.Neurology 47, 690 – 695 (1996).
[3] Huillard d’Aignaux J. et al.: Incubation period of Creutzfeldt-Jakob disease in human growth hormone recipients in France.Neurology 53, 1197 – 1201 (1999).
[4] Brown P. et al.: Iatrogenic Creutzfeldt- Jakob disease at the millennium.Neurology 55, 1075 – 1081 (2001).
[5] Ministerium für Arbeit,Gesundheit und Soziales NRW: Warnung vor gefährlichen illegalen Arzneimitteln. Pressemitteilung, 26. Februar 1997.
[6] Bayerisches Staatsministerium für Gesundheit, Ernährung und Verbraucherschutz: Warnung vor illegal erworbenen Wachstumshormonen aus Osteuropa.
Pressemitteilung Nr. 64, 5. März 2001.
[7] N.N.: Vor Fälschungen wird gewarnt. Dtsch.Apoth. Ztg.141,1652 – 1656 (2001).
[8] N.N.: Infektionen durch Doping. Der Spiegel (17), 17 (2001).
[9] Ministerium für Soziales, Familie und Gesundheit Thüringen:Akute Gefährdung durch Hormonprodukte.Pressemitteilung, 10. Juli 2002.
[10] Dingermann T.:Gentechnik,Biotechnik: Prinzipien und Anwendungen in der Pharmazie, 387 – 403.Wissenschaftliche Verlagsgesellschaft, Stuttgart 1999
[11] DeNoto F.M.,Moore D.D. and Goodman H.M.:Human growth hormone DNA sequence and structure: possible alternative splicing.Nucleic Acids Res. 9, 3719 – 3731 (1981).
[12] Chapman G.E. et al.: The 20,000 molecular weight variant of human growth hormone. J. Biol. Chem. 256, 2395 – 2401 (1981).
[13] SWISS-PROT: Somatotropin P01241, 2002.www.expasy.org/sprot.
[14] de Vos A.M.,Ultsch M.and Kossiakoff A.A.: Human growth hormone and extracellular domain of its receptor. Science 255, 306 – 312 (1992).
[15] Argetsinger L.S. et al.: Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74, 237 – 244 (1993).
[16] Cunningham B.C. et al.:Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule.Science 254,821 – 825 (1991).
[17] N.N.:Vorläufiger Rückzug eines Wachstumshormons. arzneitelegramm (5), 39 (1985).
[18] N.N.: Doping. Im Trend: Anabolika- Mix mit Wachstumshormon.Dtsch.Apoth. Ztg. 133, 980 (1993).
[19] Rauscher M. und Korte S.: Wachstumshormone – STH,IGF und Insulin. ISPVerlag, Arnsberg 2000.
[20] Dubbels W.:“Saubere Spiele”in Sydney? Dtsch.Apoth. Ztg. 140, 5435 – 5437 (2000).
[21] Schänzer W.: Dopingkontrollen und aktueller Stand der Nachweismethoden. Dtsch. Z. Sportmed. 51, 260 – 266 (2000).
[22] Wu Z. et al.: Detection of doping with human growth hormone. The Lancet 353, 895 (1999).
[23] Boos C. et al.: Medikamentenmißbrauch beim Freizeitsportler im Fitneßbereich. Dtsch.Ärztebl.95,953-957 (1998).
[24] Oldenburg A.: Fountain of youth flows from the needle.To fight the onslaught of age, baby boomers with big bucks inject human growth hormone every day.
USA Today 11/14/00 (2000). www.usatoday. com/life/health/alternative/lhalt013.htm.
[25] Lerchl A., Jockenhövel F.und Allolio B.: Hormone gegen das Altern – Möglichkeiten und Grenzen. Dtsch. Ärztebl. 98, A-2041 (2001).
[26] Koch E.:ZDF-Reportage “Die Anabolika- Connection”, gesendet im Juni 2000.
[27] Bangham D.R., Gaines Das R.E. and Schulster D.:The international standard for human growth hormone for bioassay: calibration and characterization by international collaborative study. Mol. Cell. Endocrinol. 42, 269 – 282 (1983).
[28] Europäisches Arzneibuch: Monographien Somatropin“, Somatropin zur Injektion” und “Somatropin-Lösung zur Herstellung von Zubereitungen”, in Europäisches Arzneibuch Nachtrag 2001, Amtliche deutsche Ausgabe. Deutscher Apotheker Verlag, Stuttgart 2001.
[29] Riggin R.M.,Dorulla G.K. and Miner D.J.:A reversedphase high-performance liquid chromatographic method for characterization of biosynthetic human growth hormone. Anal. Biochem. 167, 199 – 209 (1987).
[30] Lämmli U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680 – 685 (1970).
[31] Charton A. et al.: A somatropin counterfeit challenging the European Pharmacopeia monograph. Pharm. Pharmacol. Commun. 5, 61 – 66 (1999).
[32] Cholewinski M.,Lückel B.and Horn H.: Degradation pathways, analytical characterization and formulation strategies of a peptide and a protein. Calcitonine and human growth hormone in comparison. Pharm.Acta Helv. 71, 405 – 419 (1996).
[33] Frenz J.,Wu S.L. and Hancock W.S.: Characterization of human growth hormone by capillary electrophoresis. J.Chromatogr. 480, 379 – 391 (1989).
[34] Mitteilung des staatlichen Arzneimittelkontrollamtes der Republik Litauen zum Zulassungsstatus des Präparates “Somatogenum- L”, 1997.
[35] Hildebrand J.R.: Arzneimittelfälschungen in den USA.Pharm. Ind.64, 147 – 150 (2002).
[36] Serono Inc.: Serono statement regarding counterfeit Serostim®, January 22, 2001. www.seronousa.com/frame_news.html?arch ive.html.
[37] N.N.:Wachstumshormon-Präparate: Arzneimittelfälschungen in Bodybuilder- Szene. Dtsch.Apoth. Ztg.133, 2402 (1993).
[38].Schludi H.,Wolferseder E.und Zeitler K.: Arzneimittelfälschungen Dtsch.Apoth. Ztg. 140, 4971 – 4978 (2000).
[39]. Jung F., Scherges M.und Fürst P.: Illegale und gefälschte Wachstums-hormonpräparate. Dtsch.Apoth. Ztg. 142, 50 – 61 (2002).
[40].Ansomone.Recombinant Human Growth Hormone for Injection (rHGH, rDNA Orgin).M Ankebio.com 2002
[41].Girard F.Gourmelen M.:Clinical experience with Somatonorm. Acta Paediatr Scand Suppl 1986;325:29-32.
[42]. Milner R.D. Clinical expirience of somatrem: U.K. preliminary report. Acta Paediatr Scand Suppl 1986;325:25-8
[43]. Pitukcheewawont P, Schwarzbach L, Kaufmann Fr. Resumption of growth after methionyl-free human growth hormone therapy in a patient with neutralizing antibodies
to methionyl human growth hormone. J Pediatr Endocrinol Metab 2002 May; 15(5):653
[44]. Jones A.J, Department of Analytical Chemistry,Genentech Inc,South San Francisco, CA 94080, USA.
[45].Massa G,Vanderschueren-Lodeweyckx M,Bouillon R.:five-year follow-up of growth hormone antibodies in growth hormone deficient children treated with earlier with
recombinant human growth hormone:Clin Endocrinol (Oxf).1993 Feb;38(2):137-42.
[46].WHO Pharmaceuticals Newsletter, No.3, 2000.Counterfeit drugs:Data collection and the Liaison Officers network
[47].www.ergogenics.org
[48]Black market hormone was really impotence drug. Helsingin Sanomat, 31- 1-2003
[49].//www.fda.gov/ola/2002/drugimportation0725. html
This article in PDF format can be obtained at
www.gensci-china.com/gensci/body_of_Science_Growth_hormone.pdf the website of GeneScience (manufacturer of Jintropin)
- Login to post comments


