Exercise Mimetics (EM)
 Skeletal muscle has long been known as the target of several growth factors and hormones, including IGFs, steroids, thyroid and neurohypophyseal hormones, often regulating both muscle development and homeostasis in postnatal life, as summarized in classical as well as more recent reviews. Such a complex hormonal regulation is not surprising, if one considers the many diverse functions muscle exerts: mechanical force production, body temperature regulation and metabolic storage due to its protein content. Active muscle accounts for over 90% of total body energy expenditure.
Skeletal muscle has long been known as the target of several growth factors and hormones, including IGFs, steroids, thyroid and neurohypophyseal hormones, often regulating both muscle development and homeostasis in postnatal life, as summarized in classical as well as more recent reviews. Such a complex hormonal regulation is not surprising, if one considers the many diverse functions muscle exerts: mechanical force production, body temperature regulation and metabolic storage due to its protein content. Active muscle accounts for over 90% of total body energy expenditure.
Much more recent is the view of muscle as the source of several hormones [5-7] making skeletal muscle the largest endocrine gland of the organism and probably the most complex, due to the number (hundreds) of peptides constituting its secretome.
A New View of the Exercised Muscle as an Endocrine Organ
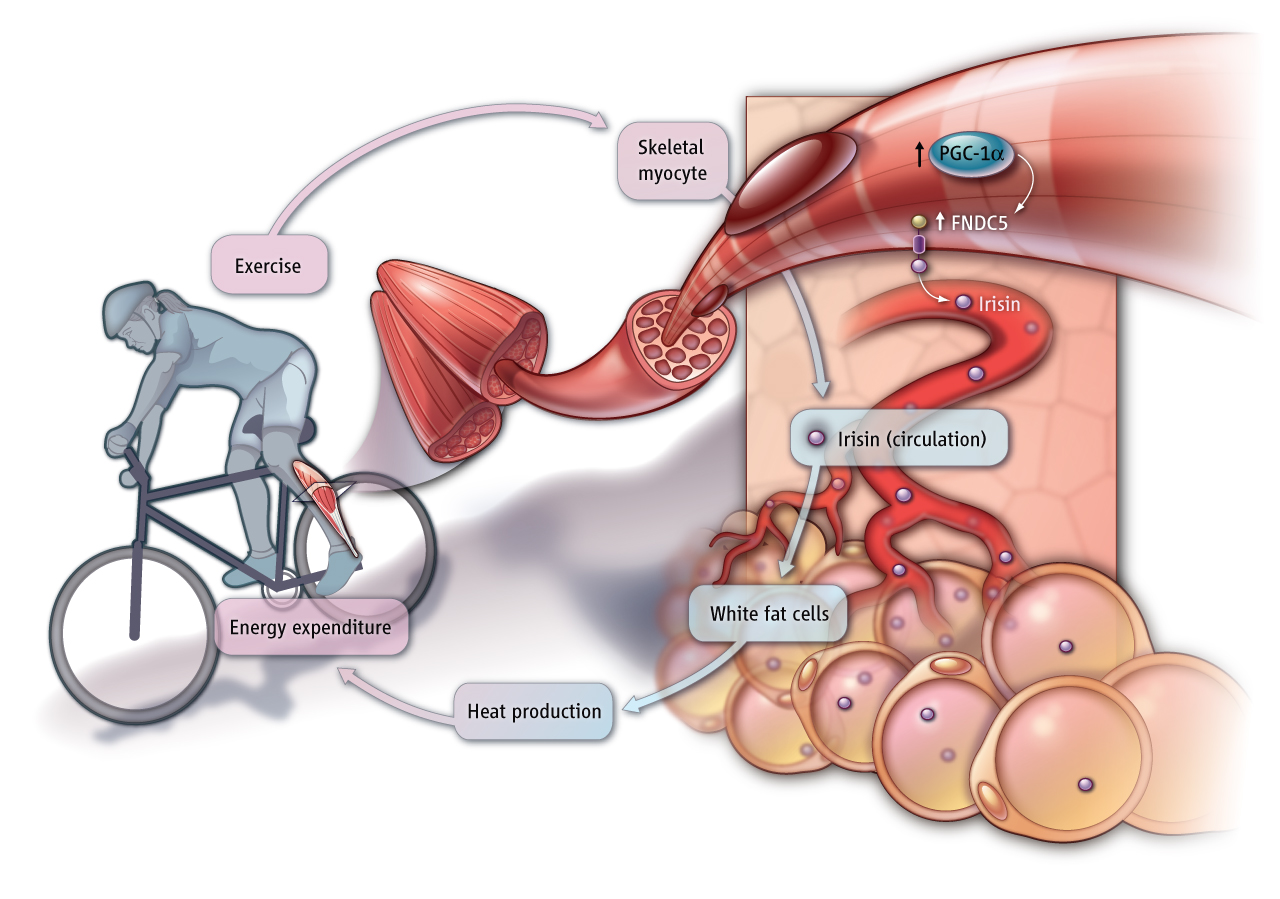
Some muscle products (myokines) have a paracrine function, regulating muscle mass (myostatin, IL-4, IL-6). Others, such as IL-8, irisin and BDNF, modulate adipose tissue metabolism, or, for example, IL-6 and additional myokines are known to act on liver, bone, immune and vascular systems. This points out to skeletal muscle, a highly vascularized organ, as one capable of affecting several targets through endocrine mechanisms. Indeed, the endocrine functions of skeletal muscle have been long suspected on the basis of clinical findings: all the abnormalities characterizing the metabolic syndrome are linked to a lack of physical activity, as are an increased risk of cancer, cardiovascular diseases and osteoporosis. Thus, exercising muscles do indeed regulate the metabolism of many distant tissues via myokines. The molecular identity of the myokines, their signaling to target tissues, the metabolic responses elicited by such signals, all contribute to a complex metabolic network which is being investigated at the molecular and physiologic levels.
Exercise Mimetics: Possible Applications and Misuses
The idea that targeting the myokine network can mimic the signals generated by exercising muscle is the rational basis for a novel family of drugs, the exercise mimetics. Exercise mimetics (EM) are a heterogeneous group of compounds that share the ability to induce pathways which are physiologically activated by exercise, thus stimulating endurance and rescuing muscle atrophy. GW1516 (also known as GW501516) or AICAR, among others, are activators of AMPK, PPARs and PGC-1, a complex of effector proteins transcription factors and co-activators. This pathway ultimately leads to the activation of both mitochondriogenesis and muscle oxidative metabolism, as it would in response to an increased AMP/ATP ratio, physiologically following exercise and energy consumption. Worth noting, EM such as GW1516 have shown to be bioactive in humans, suggesting a readily translational application for these drugs. Also the antioxidant resveratrol has been shown to synergize with exercise, positively affecting muscle performance, mitochondriogenesis and insulin sensitivity: its mechanism of action, however, which apparently goes beyond its antioxidant effect, is still unclear.
Since EM make myofibers more energy-efficient and fatigue-resistant by reducing glycogen dependency and increasing fatty acid oxidation, many possible applications in pathology are proposed, including the pharmacological treatment of cancer- and diabetes-associated cachexia and sarcopenia.
Among possible applications for the general population, EM could be used to avoid many consequences of inactivity due to aging, reduced gravity, forced immobilization or life style: those involve developing insulin resistance, fat accumulation, metabolic syndrome, type 2 diabetes, all conditions characterized by high social costs.
 It should be pointed out that, while GW1516 synergized with exercise in inducing endurance in mice, it increased muscle gene expression without significantly modifying endurance when given to sedentary mice. Conversely, AICAR was shown to both induce metabolic gene expression and enhance running endurance in sedentary mice. The use of “exercise pills” to respond to the increasingly serious problem of physical inactivity has been discussed and commented elsewhere, and the ability of EM to fully mimic exercise has been questioned. Needless to say, the toxicities of the various EM should be seriously considered and pondered in the context of an evident therapeutic indication.
It should be pointed out that, while GW1516 synergized with exercise in inducing endurance in mice, it increased muscle gene expression without significantly modifying endurance when given to sedentary mice. Conversely, AICAR was shown to both induce metabolic gene expression and enhance running endurance in sedentary mice. The use of “exercise pills” to respond to the increasingly serious problem of physical inactivity has been discussed and commented elsewhere, and the ability of EM to fully mimic exercise has been questioned. Needless to say, the toxicities of the various EM should be seriously considered and pondered in the context of an evident therapeutic indication.
A quick internet search reveals a handful of online retailers that claim to be selling GW1516, mainly, it would seem, to the bodybuilding market. And skimming through various bodybuilding forums reveals an enthusiasm among some to try the product, either in spite of or in ignorance of the dangers.
In a series of experiments in the 2000s, Ronald M. Evans from the Howard Hughes Medical Centre and Salk Institute showed that mice who were given GW1516 were able to run for twice the distance of their non-dosed counterparts — a huge boost in endurance. How is this possible?
To quote a BBC article, GW1516 appears to “have an effect on a gene that’s involved in the building and regulation of muscle” — a gene known as PPAR-delta. The research done by Evans and his colleagues showed that giving GW1516 to the mice enhanced the activity of the PPAR-delta gene, leading to the development of slow-twitch muscle that will preferentially burn fat over sugar or muscle protein. And PPAR agonists are known to be PEDs.
You can see the benefit for endurance athletes here: if exercise is burning fat rather than carbohydrates and muscle protein, then you can utilise more energy sources without losing muscle mass.
Not only that, but when GW1516 is combined with another “fitness in a pill” compound, AICAR, it can create endurance benefits far greater than either compound in isolation. AICAR activates so-called AMP-activated protein kinase (AMPK) which stimulates glucose uptake by skeletal muscle cells. The mice that were given AICAR by Evans and his team were able to run 44% further than the mice that didn’t get the drug. Most startling of all, the mice saw that 44% benefit without doing any training.
It’s for this reason that AICAR (and GW1516 as well) was heralded as “exercise in a pill” and the reason that it has potential as a performance-enhancing drug.
As enhancers of physical performance, EM treatments would be considered doping agents in sport. Indeed, in the original report by Matsakas and Narkar, AICAR amplified normal mouse response to exercise and induced greater endurance adaptation than exercise alone, thus raising concern of substance abuse by athletes. In particular, endurance performances in sports such as marathon, biking and long distance swimming could be greatly enhanced by EM. However, tests are reportedly available for detecting both GW1516 and AICAR and their metabolic by-products.
 Sweating Hard or Swallowing Pills?
Sweating Hard or Swallowing Pills?
EM could be the ideal treatment for patients who cannot have access to or need withdrawing from endurance training programs due to various circumstances. In addition, EM could be exploited to target specific metabolic pathways, which are altered in some muscle pathologies or in conditions linked to aging or forced immobility. The potential for clinical use of EM exists: GW1516 and AICAR may exert effects on sugar and lipid metabolism, thus treating or delaying the establishment of the metabolic syndrome. GW1516 can enhance the response to even moderate exercise, whereas AICAR might be used also when no exercise is possible.
However, physical exercise has systemic effects and it is highly unlikely that a single compound or pathway can mimic the complexity of exercise effects on the organism. Accordingly, the use of EM for organ failure, such as COPD, which is less heavily characterized by muscle wasting than cancer, remains speculative. Even though we cannot exclude general EM effects mediated by selective muscle stimulation, such evidence is missing to date and EM impact on different organs needs to be further elucidated. Research must proceed by better characterizing the complex network of the myokines and their mechanisms of action, and clarifying, at the molecular level, the muscle response to exercise. Furthermore, pharmacological research should strive to develop new molecules capable of interfering with those complex mechanisms with minimal toxicity, thereby pursuing important aims such as decreasing the social costs of the metabolic syndrome and contributing to cancer prevention.
GW1516 (a.k.a GW501516 or Endurobol) was first investigated by GlaxoSmithKline (GSK) who were looking to develop an “exercise in a pill” to help combat the worsening obesity epidemic. The drug passed pre-clinical studies and was allowed to be tested in humans for Phase I, II and IV trials.
The trials delivered some success but when high-doses of the drug were linked with increased rates of cancer in animals, GSK decided to scrap further development of the drug for humans. To quote a GSK representative, “toxicities were found in routine, long-term animal studies that were being conducted in parallel with the clinical studies.”
Quote from New Scientist: “tests on rats showed that at all doses, the drug rapidly causes cancers in a multitude of organs, including the liver, bladder, stomach, skin, thyroid, tongue, testes, ovaries and womb.”
 On the discussion-forums bodybuilders emphasized that this were huge doses for a long time (104 weeks). They translated these doses to a 100 kg guy and just multiplied the doses used on rats mg/kg/day with 100.
On the discussion-forums bodybuilders emphasized that this were huge doses for a long time (104 weeks). They translated these doses to a 100 kg guy and just multiplied the doses used on rats mg/kg/day with 100.
Then the abused doses would be indeed ridiculous, but that is not how it should be calculated:
FASEB J. 2008 Mar;22(3):659-61.
If you want to assume that if a dose is carcinogenic (causes cancer) in rodents it will also cause cancer in humans then a dose of 5 mg/kg in a rat equals 81 mg in a 100 kg bodybuilder. The normal dose bodybuilders take is 10-20 mg pre-workout.
And in 2015?
Miracle Weight Loss Pill "Irisin" Allows For Easy Workouts.
Scientists claim they've created a pill that provides a workout without the sweat
Good news for couch potatoes: Researchers are developing a drug that provides all the benefits of exercise, with no actual workout required, according to the New York Times.
In a study published in Nature Medicine, researchers injected a compound into obese mice that increased production of the muscle protein REV-ERB, which is known to have an impact on sleep and on animals’ internal biological clocks.
 The animals injected with the compound lost weight and improved their cholesterol levels – even when on a high-fat diet, the New York Times reported. Furthermore, the injected mice also used more oxygen during the day and expended 5 percent more energy compared to mice in the control group – even though they were not exercising more.
The animals injected with the compound lost weight and improved their cholesterol levels – even when on a high-fat diet, the New York Times reported. Furthermore, the injected mice also used more oxygen during the day and expended 5 percent more energy compared to mice in the control group – even though they were not exercising more.
Researchers also examined the effect that REV-ERB had on muscles by engineering a test group of mice designed to express abnormally low levels of REV-ERB. This group of engineered mice were highly un-athletic, exhibiting very low levels of endurance and displaying a maximum oxygen capacity approximately 60 percent lower than that of normal mice.
 However, when researchers injected these mice with the compound, they were able to stimulate the production of REV-ERB, strengthening the muscles of the weakened mice, according to the New York Times.
However, when researchers injected these mice with the compound, they were able to stimulate the production of REV-ERB, strengthening the muscles of the weakened mice, according to the New York Times.
Additionally, when researchers injected the compound into sedentary mice, the animals were then able to run longer and for greater distances than untreated mice.
The drug "certainly seems to act as an exercise mimic," study co-author Thomas Burris, chairman of the department of pharmacological and physiological science at St. Louis University School of Medicine, said.
Though it is still unclear whether or not the drug can be safely used in humans, researchers hope it will someday be used to help disabled people experience some of the health benefits of exercise.


