“Designer steroids”
 Designer steroids are anabolic steroids or other steroid-like performance-enhancing drugs designed to be undetectable in drug testing. Although anabolic steroids are banned in most athletic organizations and contests worldwide, some athletes have nevertheless sought ways around the bans. Anabolic steroids mimic the effect of male sex hormones on the body, and whether someone is using them can usually be determined by blood or urine tests. Athletes looking for the performance-enhancing benefits of steroids, without being caught by testing, have created a demand for undetectable steroids. This has led some chemists or scientists to research and produce synthetic designer steroids for sale.
Designer steroids are anabolic steroids or other steroid-like performance-enhancing drugs designed to be undetectable in drug testing. Although anabolic steroids are banned in most athletic organizations and contests worldwide, some athletes have nevertheless sought ways around the bans. Anabolic steroids mimic the effect of male sex hormones on the body, and whether someone is using them can usually be determined by blood or urine tests. Athletes looking for the performance-enhancing benefits of steroids, without being caught by testing, have created a demand for undetectable steroids. This has led some chemists or scientists to research and produce synthetic designer steroids for sale.
The list of anabolic steroids banned for use by elite athletes has a long history dating back to 1974. The lists were for a long time prepared by the International Olympic Committee and then, with the formation of the World Anti-Doping Agency (WADA), the duty of deciding which substances should be banned passed on to WADA. Lists in general are finite entities requiring individual substances to be added as needed. This can be a slow process, especially when lists are reviewed once a year or less. The IOC overcame this problem by including the phrase “and related compounds” early in developing their list. This phrase later became “related pharmacologically and chemically compounds” in order to cover substances that acted in a similar manner to anabolic steroids but which would have required considerable argument about whether or were not they were related to anabolic steroids. The issue of b2-agonists fell into such a void when it was realized that they were being abused within the European Union to increase lean muscle mass in animals in order to increase meat production and that this practice had spilled over into human sport. This prompted a change in the wording of the list from “anabolic steroid” to “anabolic agent”. There occasionally occur new substances which are only covered by the term “pharmacologically related”. These substances may have quite different chemical structures but are being developed for anabolic effects, e.g. SARMs (which were added to the WADA list in 2008) and so there will be need to add them to the WADA Prohibited List as individual substances or classes. Until they are added to the list, the coverage by the “related pharmacologically and chemically compounds” phrase will need to suffice. The inclusion of this phrase was an acknowledgement by the administrative bodies, even back in the very early stages in the anti-doping struggle, that science is constantly changing and new drugs are continuously being discovered and that such discoveries can be put to unintended purposes.
 The “Designer Anabolic Steroid Control Act 2014”, seeks to close a loophole exploited by steroid sellers who spike bodybuilding supplements with chemically tweaked compounds. The bill targets bodybuilding products, often marketed as dietary supplements, that can be found in stores and on the Internet claiming to be all-natural muscle-builders when they actually contain chemically altered versions of anabolic steroids. This bill would add 27 known anabolic steroids to the DEA's list of controlled substances — helping to bring it up-to-date. It would also give the DEA the authority, as it identifies new designer steroids similar to those already on its list, to quickly add them temporarily, allowing action until the compound is permanently added to the list.
The “Designer Anabolic Steroid Control Act 2014”, seeks to close a loophole exploited by steroid sellers who spike bodybuilding supplements with chemically tweaked compounds. The bill targets bodybuilding products, often marketed as dietary supplements, that can be found in stores and on the Internet claiming to be all-natural muscle-builders when they actually contain chemically altered versions of anabolic steroids. This bill would add 27 known anabolic steroids to the DEA's list of controlled substances — helping to bring it up-to-date. It would also give the DEA the authority, as it identifies new designer steroids similar to those already on its list, to quickly add them temporarily, allowing action until the compound is permanently added to the list.
The bill doesn't address the growing problem of designer stimulants, including amphetamine-like and methamphetamine-like compounds, that have been detected over the past year in several mainstream sports supplements. There have been tests finding these compounds in a popular pre-workout powder called Craze, as well as other products. I also posted about it here. The same goes for designer peptides, this problem is only very recently been discussed on this blog.
Everything that gets banned is still produced. You just need to find a lab that makes it, or a source that sells it. These bills/bans don't really do much, it won't help society, it won't do anything except push more people into the black market.
BALCO, an Insight into Conspiracy
 The term “designer steroid” appears to have been coined at the time of the BALCO investigations. These occurred on 23 September 2003 when the Bay Area Laboratory Co-Operative (BALCO), owned by Victor Conte, was raided by agents from the US Internal Revenue Service Criminal Investigations Unit and the San Mateo County Narcotics Task Force. Records and materials were seized in that raid, and subsequent investigations revealed that Barry Bonds – a baseball record-breaking hitter, Kelli White – a double world sprint champion, Marion Jones – triple Olympic champion and Tim Montgomery – world 100 m record holder were listed as BALCO clients.
The term “designer steroid” appears to have been coined at the time of the BALCO investigations. These occurred on 23 September 2003 when the Bay Area Laboratory Co-Operative (BALCO), owned by Victor Conte, was raided by agents from the US Internal Revenue Service Criminal Investigations Unit and the San Mateo County Narcotics Task Force. Records and materials were seized in that raid, and subsequent investigations revealed that Barry Bonds – a baseball record-breaking hitter, Kelli White – a double world sprint champion, Marion Jones – triple Olympic champion and Tim Montgomery – world 100 m record holder were listed as BALCO clients.
A prelude to this event occurred in 2002 when the UCLA WADA accredited laboratory led by Professor Don Catlin published the discovery of a new steroid, norbolethone, that appeared in the sporting arena. The discovery of this compound was triggered by the appearance of a number of urine samples within that laboratory which showed extremely suppressed endogenous steroid profiles. Normal urines have a set of endogenous steroids that can be used as markers of normal steroid biosynthesis. These include testosterone (T) and epitestosterone (E) as well as their end metabolites, androsterone and etiocholanolone. The latter two normally occur at a high concentration.
Classified documents saved after the collapse of the German Democratic Republic revealed that, since 1983, a pharmaceutical company had produced preparations of epitestosterone propionate exclusively for the governmental doping programme. Epitestosterone, an epimer of testosterone, is a steroid with no anabolic activity but its administration with testosterone simultaneously or sequentially enables an athlete to manipulate the test for testosterone administration if the test is based solely on determination of the urinary testosterone/ epitestosterone (T/E) ratio.
In the samples delivered to the laboratory these endogenous steroids were present at a low concentration without the urines being classified as dilute as determined by specific gravity measurements. Seeing unusually large numbers of these types of urine sample, the laboratory became suspicious that the athletes were taking something that suppressed steroid production. Further investigation of several urine samples allowed the detection and confirmation that the causative compound was norbolethone. The detection of this substance became routine worldwide. Knowing this, athletes appear to have never used it again.
Sometime before the raid on the BALCO premises the same UCLA laboratory received a syringe containing an oily material. The laboratory undertook a considerable amount of work to show that this oil contained another new steroid which they termed tetrahydrogestrinone (THG) . The discovery of this substance was announced to the world through the United States Anti-Doping Agency (USADA) in September 2003.
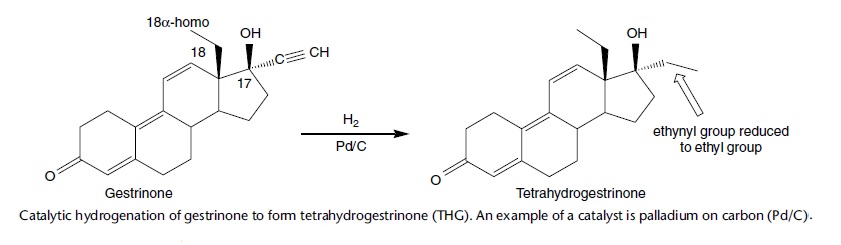
After the raid on BALCO, records showed that they were responsible for distributing the THG as a material called “Clear” which had been supplied to them through a chemist called Patrick Arnold.
Tetrahydrogestrinone can be easily manufactured by the catalytic hydrogenation of the ethynyl group of the progestogen gestrinone. This relatively simple synthetic step hides the thinking that probably lay behind the design of THG. Given the close homology of their receptors, there is an overlap between the activity of progestogens and androgens, especially those xenobiotic steroids that lack the C-19 methyl group, but which activity predominates depends on whether the alkyl substituent at carbon-17 is ethynyl or ethyl. Substitution of the 17a-H with an ethynyl group on nandrolone, a 19-nor anabolic steroid with some progestational activity, will result in a potent orally active progestogen, this being called norethisterone (norethindrone), a steroid that is still used in some contraceptives today. The synthetic route is described in a seminal paper by Djerassi et al. (1954).
However, substitution with an ethyl group on nandrolone rather than ethynyl group results in another anabolic steroid known as norethandrolone, which also has oral activity. Gestrinone, is a pharmaceutically available progestogen that lacks the C-19 angular methyl group but has a 17a-ethynyl group, and it follows that reduction of this ethynyl group to the tetrahydro product should make THG a ‘potent’ androgen. This is indeed the case, as subsequently THG was found to be a highly potent androgen (and progestogen) in an in vitro bioassay system expressing human steroid receptors (Death et al., 2004), and it promotes muscle accretion in orchidectomized male rats .
Despite the presence of the 17a-alkyl function, which should make the steroid resistant to first-pass metabolism, it is of interest that the instructions from BALCO Laboratories were to place a few drops of the liquid preparation under the tongue, that is, a sublingual route of administration. THG was invisible on the routine gas chromatography–mass spectrometry screen employed by the WADA-accredited laboratories and necessitated the development of a liquid chromatography–mass spectrometry/mass spectrometry (LC–MS/MS) screen for its detection
Underground chemists appear also to be accessing information concerning other steroids that were synthesized several decades ago by pharmaceutical companies but were never marketed. Such steroids that have been detected until recently are norbolethone , which was reputed to have been the active ingredient of ‘The Clear’ before being replaced by THG, and madol, which is also referred to as desoxymethyltestosterone. Although the extent of this activity appears to be limited, as screening procedures rely on targeting selecting ions for monitoring by mass spectrometry, unknown steroids may escape detection. To demonstrate how this problem may be addressed, Thevis et aldeveloped an LC–MS/MS screening method based on common fragmentation pathways and Nielen et al. used a combination of androgen bioassay detection and electrospray quadrupole time-of-flight mass spectometric identification
The effects of the BALCO scandal were far-reaching within anti-doping. The directors of BALCO and the chemist Patrick Arnold were prosecuted for their part in this conspiracy and were given sentences which did include a short period in detention.
The compounds uncovered in the BALCO incident, as well as other substances sold as “supplements”, often appear to have been synthesized and published many years ago (see later the part on SuperDrol). As only limited data about biological activity is available, it may be of interest to see what discoveries were made in the early history of the drug industry investigating androgenic activity and the information available about their usefulness as potential designer steroids. The published data often only lists the substances as having anabolic or anti-estrogenic activities using a particular animal or in vitro model. No information on toxicity or effects in humans is available for many published structures.
Anabolic Steroid Chemistry
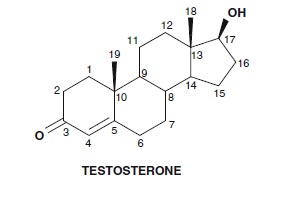 Anabolic steroid structures are based on the male hormone testosterone, which is the main steroid responsible for the biological activity. The testosterone molecule shown left, has a number of positions which can be modified by addition/ removal of double bonds, reduction of the keto group, and substitution using heteroatoms, halogens or addition of functional groups such as hydrocarbon chains and heterocyclic rings. Some common anabolic steroids banned for many years by the IOC and then WADA are shown in Fig. 2 to illustrate the successful changes that have been made and the drugs which have been brought to the medical field for use by androgen-deficient individuals. It is only when these drugs are abused by healthy athletes that an issue of chemical cheating occurs. The compounds linked to the BALCO scandal such as THG and norbolethone are shown underneath. These are shown together with trenbolone and gestrinone to highlight the close relationship between these molecules. This type of related compound synthesis is common in pharmaceutical chemistry where many modifications are made to an original active structure to generate new drugs with the desired or improved activity. The structure relationships for the activity are then mapped by testing the prepared compounds and finding the changes that retain the desired actions.
Anabolic steroid structures are based on the male hormone testosterone, which is the main steroid responsible for the biological activity. The testosterone molecule shown left, has a number of positions which can be modified by addition/ removal of double bonds, reduction of the keto group, and substitution using heteroatoms, halogens or addition of functional groups such as hydrocarbon chains and heterocyclic rings. Some common anabolic steroids banned for many years by the IOC and then WADA are shown in Fig. 2 to illustrate the successful changes that have been made and the drugs which have been brought to the medical field for use by androgen-deficient individuals. It is only when these drugs are abused by healthy athletes that an issue of chemical cheating occurs. The compounds linked to the BALCO scandal such as THG and norbolethone are shown underneath. These are shown together with trenbolone and gestrinone to highlight the close relationship between these molecules. This type of related compound synthesis is common in pharmaceutical chemistry where many modifications are made to an original active structure to generate new drugs with the desired or improved activity. The structure relationships for the activity are then mapped by testing the prepared compounds and finding the changes that retain the desired actions.
Both norbolethone and THG are related to the anabolic agent gestrinone which is available as a pharmaceutical product. Gestrinone has weak androgen and progestogen activity. It also possesses anti-estrogen activity. THG on the other hand had been reported as having very potent anabolic activity. Since gestrinone is available as a raw material it 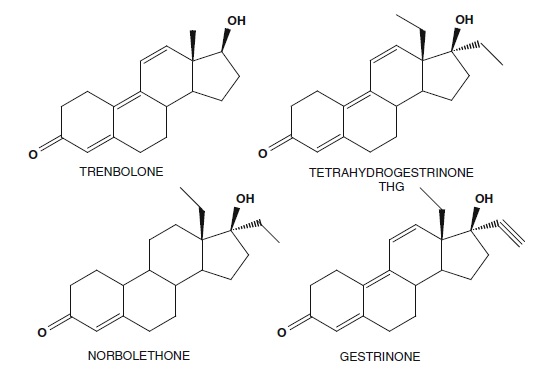 may be a good starting material for synthesis of both norbolethone and THG. THG was prepared by hydrogenation of the acetylenic function at C17 in gestrinone and separation of the THG from dihydrogestrinone. Norbolethone was originally synthesised as part of a total steroid synthesis. It was available as a pharmaceutical in the 1960s but was discontinued and is no longer commercially available. It has been reported that norbolethone is more potent as an anabolic agent than testosterone propionate and also less androgenic (Albanese et al. 1968). These are structurally similar to other known androgens such as trenbolone and nandrolone.
may be a good starting material for synthesis of both norbolethone and THG. THG was prepared by hydrogenation of the acetylenic function at C17 in gestrinone and separation of the THG from dihydrogestrinone. Norbolethone was originally synthesised as part of a total steroid synthesis. It was available as a pharmaceutical in the 1960s but was discontinued and is no longer commercially available. It has been reported that norbolethone is more potent as an anabolic agent than testosterone propionate and also less androgenic (Albanese et al. 1968). These are structurally similar to other known androgens such as trenbolone and nandrolone.
This relationship between gestrinone and other “designer” steroids is just one example of the types of modifications that have been based on the testosterone molecule. Early literature from the 1950s and 1960s shows numerous such examples together with data for the anabolic effects that were achieved. The literature does not however indicate why these substances were not introduced into the marketplace, especially since some had promising activities. The chemist now wishing to produce designer steroids can review these papers and find substances that will not be screened for. Of course this approach will be undertaken with almost no knowledge of the effects that the drug may have, both “beneficial” and adverse. The lesson from BALCO, where drugs were produced with very little or no appropriate human or animal studies being undertaken, is that this ethical consider-ation does not appear to be an issue and many steroids will be sold using athletes as the guinea pigs.
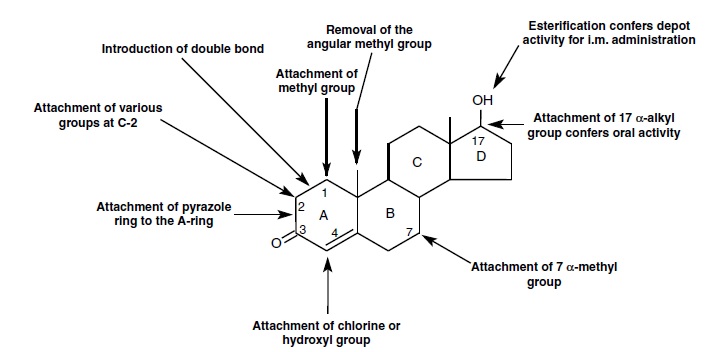
The design of new steroids is relatively simple. It’s also possible to modify pro-hormones. A similar process can be used to speculate about new designer steroids as is shown below.
New designer steroids may have for instance:
- an extra methyl group, mostly on C7, C1, C2 and of course on C17, but possibly also on C4 and C6.
- an extra ethyl group, mostly on C13 and C17.
- more extra methyl or ethyl groups on combinations of the positions mentioned above.
- no methyl group on C19, possibly in combination with extra methyl or ethyl groups elsewhere in the molecule.
These are speculations about addition or omission of extra methyl or ethyl groups but other possibilities are:
- the introduction of one or more extra double bonds.
- the introduction of an extra hydroxyl or carbonyl group.
- the introduction of an extra F or Cl atom.
These are familiar variations, which all have been tried by chemists in the past, but not on every known steroid. There are of course many other substituents that can be introduced on basically all places in the steroid, but we will not go into that. Experience has learned that many variations in the steroid are tolerated with preservation of most of its activity, but big changes are not allowed.
It is not clever to introduce variations in the structure of a new designer steroid, that ban be converted by enzymes in the body in some known anabolic steroid. This immediately leads to positive doping tests. The designer steroid is then just a pro-hormone. New designer steroids must be designed in such a way that also their metabolites are unknown steroids.
Early studies showed that certain functional groups within the testosterone molecule were essential for androgenic and anabolic activity (Klimstra 1969; Kochakian 1950, 1976). The 17b-hydroxyl group was essential but could be esterified for administration by intra-muscular injection and the biological proper-ties were enhanced and prolonged simply due to reduction in the rate of elimination due to the slow release of the parent non-esterified steroid. Esters that are commonly used include the decanoate, undecanoate, propionate, enanthate and cypionate. Similarly the presence of the 4,5 double bond gave an increase in activity while reduction of the 3-keto group increased the androgenic activity but not the anabolic activity. However, removal of the 3-keto function while retaining the 4,5 double bond can retain anabolic activity, e.g. ethylestrenol (Fig. 4) marketed as Maxibolin (used in veterinary medicine). This compound can have an oxygen function reintroduced on metabolism to give 19-norandrosterone as well as meta-bolites of norethandrolone.
Removal of the 19-methyl group in testosterone led to the development of the 19-nor steroids such as nandrolone. It was apparently found to be as myotrophic as testosterone but with greatly reduced androgenic activity. In many cases similar functional changes to give analogues similar to those made for testosterone were studied for nandrolone leading to development of drugs such as norethandrolone (Nilevar), the 17a-ethyl analogue . The 5a stereochemistry at C5 is also important for retaining biological activity. For example, dihydrotestosterone (DHT), which is a metabolite of testosterone and is applied as a skin cream preparation such as Andractim, has the 5a stereochemistry and is considerably more potent than testosterone. However, other saturated meta-bolites such as androsterone and its diol analogue are not active.
Compounds which had a double bond introduced into the C1,2 position retained activity. The most orally active of these was methandienone (methandrostenolone, Dianabol). It is interesting that methandienone is still one of the most popular steroids in use today, giving rise to many “positive” test results. This is surprising since Ciba stopped manufacture of the agent in 1982; it means that it is available via an underground process, with many sources from Russia, China, Thailand and India. Addition of more double bonds into the 19-nor steroid molecule produced compounds such as methyltrienolone, trenbolone, gestrinone and recently the designer steroid THG. Methyltrienolone has not appeared as a drug for animal or human use but is often used as the reference point for in vitro biological activity. Another steroid, advertised as methyldienolone, appeared briefly on the “supple-ment” market but the product was suspect with no methyldienolone present in the formulation. It did contain numerous related unsaturated steroids, probably from poor manufacture.
Looking at the list of steroids that are in use today and those studied but not used commercially, it can be seen that many substituents can be added and activity retained. The compounds with addition of alkyl groups at C17 (methyl, ethyl, ethynyl) occur in preparations such as methyltestosterone, ethylestrenol and gestrinone. Addition of a methyl group at C7, C2, C1 gives compounds such as MENT (7a-methyl-19-nortestosterone), drostanolone and mesterolone . MENT has very high biological activity (Dorfman 1968) and while it was studied as a possible male contraceptive (Nieschlag and Behre 2004) it has not been marketed and is very difficult to obtain. The analogous 7a-methyldihydrotestosterone was found to be more active as an androgen than testosterone. The 6a-methyltestosterone has low activity. The analogue, 6a-methylan-drostenedione, has been found in “supplements” together with androstenedione. Methylation in the 18 position has also produced compounds with pronounced anabolic activity. These 13-ethylgonan derivatives occur in several birth control pills having estrogen/progestin activity, e.g. levonorgestral, and have structures similar to closely related compounds THG, gestrinone and norbolethone while also have androgenic activity. In fact, this tight inter-relationship between anabolic steroids, estrogens and progestins is often seen through side effects from anabolic steroid use where some estrogenic metabolites give rise to female characteristics. The development of these side effects in bodybuilders (and other athletes that have used steroids) have led to the sale of compounds which have anti-estrogen properties and which are publicized for use by steroid users to be taken at the same time to counteract unwanted effects. An example of this is the underground recommendation to take substances such as tamoxifen with steroids and this in turn led to the banning of compounds with anti-estrogenic properties for use in sport.
The effect of addition of heteroatoms has been investigated but few compounds have reached the pharmaceutical market. The successful steroids have either chlorine or fluorine as substituents at the 4 or 9 position, such as in dehydrochlor-omethyltestosterone (DHCMT, oral-Turinabol), fluoxymesterone (Halotestin), clostebol (as the acetate, Alfa-Trofodermin; Clostene; Megagrisevit; Steranabol, and 19-norclostebol (as acetate). DHCMT, which was developed for clinical use, was extensively used by the East German athletes in a state-controlled doping programme. Even in 2007 there is litigation against Jenapharm by some athletes from that programme for compensation for serious medical problems – infertility among women, embarrassing hair growth, breast cancer, heart problems and testicular cancer. An estimated 800 athletes developed serious ailments including a female shot-putter who took so many male hormones she decided to have a sex change. This scandal appears to be one of the first conspiracies using designer steroids, since DHCMT was developed for the East German programme and its problems are still manifest more than 50 years later. An expansive list of chlorinated and other 6-chloro substituted steroids and their activities has been published by Weichert et al. , with many mainly having anti-androgenic activity.
Compounds with heterocyclic functional groups fused to the A ring are used as orally active anabolic steroids, some of which are still taken by sports persons even though they have been detectable for 20 years. The steroid stanozolol (Winstrol) was made famous by the Canadian runner Ben Johnson when he was found to have it in his system during the 1988 Seoul Olympics. This molecule contains the pyrazole ring fused to positions C2 and C3 and a 17a-methyl group and has very high anabolic activity, remaining a steroid “of choice” even today. The oral form is marketed for human use whereas an aqueous suspension for injection is used in the veterinary field. Much of the veterinary preparation is diverted for use by athletes for muscle development. A modification to the structure of stanozolol was made by introducing the 4,5 double bond to give BAS-71 and this compound retained activity. Recently the stanozolol analogue without the 17-methyl group has been sold on the “supplement” market as Prostanazol . Another such steroid, furazabol (Neo-ponden), has an isoxazole ring instead of the pyrazole ring, but is an uncommon steroid which was only used in Japan. The 17b-hydroxy analogue (without the 17a-methyl group) also had activity but has so far not been used in the pharmaceutical industry. The furazabol derivatives with a 16-methylene, 16b-methyl, 16a and 16b-hydroxy functional group were prepared but “did not exhibit anabolic activity comparable to that of the parent compound”. Stanozolol appears to be about twice as potent as methyltestosterone but furazabol was 29 times as active orally in producing nitrogen retention in animals.
Other fused rings that have been prepared as part of steroid structure activity research include thiazoles, pyridines, pyrimidines, pteridines, oxadiazoles and pyrroles. While these may have lower activity it may still be possible that unscru-pulous marketers may make and sell some of these substances.
The A ring 2,3-thio-epoxide (10275-S, 2a,3a-epithio-5a-androstane-17b-ol, Fig. 7) was found to have anti-oestrogenic activity (which caused delayed implantation in the intact rat), an activity which was found to be surprisingly long-lived. It also has androgenic and myogenic activity. Compounds with an A nor-heterocycle system have also been investigated. These compounds related to 2-thia-A-nor-5a-androstane-17b-ol included heteroatoms of oxygen, selenium, tellurium disulphide, sulphone, sulphoxide. The sulphur and selenium analogues were active while the others were inactive (see Fig. 7). Further studies on A ring homosteroids containing a heteroatom showed that the sulphur derivative 3-thia-A-homo-5a-androstane-17b-ol (Fig. 7) was the most potent (Zanati and Wolf 1972), having similar activity to testosterone. The conclusion from these studies was that steric properties were dominant factors for biological activity. This was also found in the series of A ring oxa steroids in which the compound 2-oxa-5a-androstan-17b-ol (Fig. 7) was the most active.
Substitution at C7 with alkylthio functions did not increase activity but did appear to improve myotropic activity relative to androgenic activity, especially in the 7a-thioethyl analogue of stanozolol (7a-ethylthio-17b-hydroxy-17a-methyl-5a-androstano[3,2-c]pyrazole, 7a-ethylthiostanozolol). Other 7-thio-steroids such as spironolactone have quite different uses such as a diuretic, but also have anti-androgen activity due to their binding to the androgen receptor.
Other unusual modifications of the steroid molecule have led to a series of 5, 7-cyclosteroids such as 17b-hydroxy-17a-methyl-5,7b-cyclo-5b-androstan-3-one . The activity was not determined. The B-homo steroid Ba 36644 (A-nor-B-homo-7a-17a-dimethylestran-17b-ol-3,6-dione (Fig. 8) has been found to be very active but does not seem to have ever been used other than as an experimental drug. Recent studies into novel steroids show that interest has not waned, with production of the 13,14-seco steroids such as 17-hydroxy-13,14-seco-androst-4-en-3-one and a cyclobutane containing compound, but any activity data has not been reported yet.
This short pre´cis of some of the anabolic steroid analogues that have been prepared clearly shows that many interesting compounds exist but with very little detailed published data on their biological activity other than some animal or in vitro testing for androgen-related mode of action. Data on activities such as toxicity and mutagenicity, which are required to be well documented in animal models before a drug can even be considered for human trials, is not available. Dealers in steroids, including those steroids present in the so-called supplements, are only interested in monetary gain and are not interested in long-term effects, or the fact that little or nothing is known about the molecule they are selling. The literature gives clues to a large variety of promising candidates for clandestine use. Most of the steroids studied in the past that have not been progressed to pharma-ceutical products, have some limited information published but do not have impor-tant animal and human toxicity and side effect data published (or even had them undertaken), and so the studies are actually being conducted on the gullible/ desperate community that buys products containing designer steroids. Thus, the only output of information on effects may appear in bodybuilding chat rooms and then in only barely understandable terms. Data for many compounds relating to oestrogenic, anti-oestrogenic or even anti-anabolic effects is not known, so pro-blems such as those currently facing the older doped East German athletes will undoubtedly occur. This assumes that these modifications to the steroid molecule do not introduce an acutely toxic or life-threatening effect. in which case the data will be in the obituary columns. The athletes that take them may or may not be aware of these issues and they use them because they believe that they are not detectable.
Surprising since Ciba stopped manufacture of the agent in 1982; it means that it is available via an underground process, with many sources from Russia, China, Thailand and India. Addition of more double bonds into the 19-nor steroid molecule produced compounds such as methyltrienolone, trenbolone, gestrinone and recently the designer steroid THG. Methyltrienolone has not appeared as a drug for animal or human use but is often used as the reference point for in vitro biological activity. Another steroid, advertised as methyldienolone, appeared briefly on the “supplement” market but the product was suspect with no methyldienolone present in the formulation. It did contain numerous related unsaturated steroids, probably from poor manufacture.
Looking at the list of steroids that are in use today and those studied but not used commercially, it can be seen that many substituents can be added and activity retained. The compounds with addition of alkyl groups at C17 (methyl, ethyl, ethynyl) occur in preparations such as methyltestosterone, ethylestrenol and gestrinone. Addition of a methyl group at C7, C2, C1 gives compounds such as MENT (7a-methyl-19-nortestosterone), drostanolone and mesterolone . MENT has very high biological activity and while it was studied as a possible male contraceptive it has not been marketed and is very difficult to obtain. The analogous 7a-methyldihydrotestosterone was found to be more active as an androgen than testosterone. The 6a-methyltestosterone has low activity. The analogue, 6a-methylan-drostenedione, has been found in “supplements” together with androstenedione (Parr et al. 2008). Methylation in the 18 position has also produced compounds with pronounced anabolic activity. These 13-ethylgonan derivatives occur in several birth control pills having oestrogen/progestin activity, e.g. levonorgestral, and have structures similar to closely related compounds THG, gestrinone and norbolethone while also have androgenic activity . In fact, this tight inter-relationship between anabolic steroids, oestrogens and progestins is often seen through side effects from anabolic steroid use where some oestrogenic metabolites give rise to female characteristics. The development of these side effects in bodybuilders (and other athletes that have used steroids) have lead to the sale of compounds which have anti-oestrogen properties and which are pub-licised for use by steroid users to be taken at the same time to counteract unwanted effects. An example of this is the underground recommendation to take substances such as tamoxifen with steroids and this in turn led to the banning of compounds with anti-oestrogenic properties for use in sport.
The effect of addition of heteroatoms has been investigated but few compounds have reached the pharmaceutical market. The successful steroids have either chlorine or fluorine as substituents at the 4 or 9 position, such as in dehydrochlor-omethyltestosterone (DHCMT, oral-Turinabol), fluoxymesterone (Halotestin), clostebol and 19-norclostebol (as acetate). DHCMT, which was developed for clinical use was extensively used by the East German athletes in a state-controlled doping programme. Even in 2007 there is litigation against Jenapharm by some athletes from that programme for compensation for serious medical problems – infertility among women, embarrassing hair growth, breast cancer, heart problems and testicular cancer. An estimated 800 athletes developed serious ailments including a female shot-putter who took so many male hormones she decided to have a sex change. This scandal appears to be one of the first conspiracies using designer steroids, since DHCMT was developed for the East German programme and its problems are still manifest more than 50 years later. An expansive list of chlorinated and other 6-chloro substituted steroids and their activities has been published by Weichert et al. ( 1967), with many mainly having anti-androgenic activity.
Compounds with heterocyclic functional groups fused to the A ring are used as orally active anabolic steroids, some of which are still taken by sports persons even though they have been detectable for 20 years. The steroid stanozolol (Winstrol) was made famous by the Canadian runner Ben Johnson when he was found to have it in his system during the 1988 Seoul Olympics. This molecule contains the pyrazole ring fused to positions C2 and C3 and a 17a-methyl group and has very high anabolic activity, remaining a steroid “of choice” even today. The oral form is marketed for human use whereas an aqueous suspension for injection is used in the veterinary field. Much of the veterinary preparation is diverted for use by athletes for muscle development. A modification to the structure of stanozolol was made by introducing the 4,5 double bond to give BAS-71 and this compound retained activity. Recently the stanozolol analogue without the 17-methyl group has been sold on the “supplement” market as Prostanazol . Another such steroid, furazabol, has an isoxazole ring instead of the pyrazole ring , but is an uncommon steroid which was only used in Japan. The 17b-hydroxy analogue (without the 17a-methyl group) also had activity but has so far not been used in the pharmaceutical industry. The furazabol derivatives with a 16-methylene, 16b-methyl, 16a and 16b-hydroxy functional group were prepared but “did not exhibit anabolic activity comparable to that of the parent compound”. Stanozolol appears to be about twice as potent as methyltestosterone but furazabol was 29 times as active orally in producing nitrogen retention in animals Other fused rings that have been prepared as part of steroid structure activity research include thiazoles, pyridines, pyrimidines, pteridines, oxadiazoles and pyrroles. While these may have lower activity it may still be possible that unscru-pulous marketers may make and sell some of these substances.
The A ring 2,3-thio-epoxide (10275-S, 2a,3a-epithio-5a-androstane-17b-ol, Fig. 7) was found to have anti-oestrogenic activity (which caused delayed implan-tation in the intact rat), an activity which was found to be surprisingly long-lived.
It also has androgenic and myogenic activity. Compounds with an A nor-heterocycle system have also been investigated. These compounds related to 2-thia-A-nor-5a-androstane-17b-ol included heteroatoms of oxygen, selenium, tellurium disulphide, sulphone, sulphoxide. The sulphur and selenium analogues were active while the others were inactive. Further studies on A ring homosteroids containing a heteroatom showed that the sulphur derivative 3-thia-A-homo-5a-androstane-17b-ol was the most potent , having similar activity to testosterone. The conclusion from these studies was that steric properties were dominant factors for biological activity. This was also found in the series of A ring oxa steroids in which the compound 2-oxa-5a-androstan-17b-ol (Fig. 7) was the most active (Zanati and Wolf 1971).
Substitution at C7 with alkylthio functions did not increase activity but did appear to improve myotropic activity relative to androgenic activity, especially in the 7a-thioethyl analogue of stanozolol (7a-ethylthio-17b-hydroxy-17a-methyl-5a-androstano[3,2-c]pyrazole, 7a-ethylthiostanozolol). Other 7-thio-steroids such as spironolactone have quite different uses such as a diuretic, but also have anti-androgen activity due to their binding to the androgen receptor.
Other unusual modifications of the steroid molecule have led to a series of 5, 7-cyclosteroids such as 17b-hydroxy-17a-methyl-5,7b-cyclo-5b-androstan-3-one . The activity was not determined. The B-homo steroid Ba 36644 (A-nor-B-homo-7a-17a-dimethylestran-17b-ol-3,6-dione (Fig. 8) has been found to be very active but does not seem to have ever been used other than as an experimental drug. Recent studies into novel steroids show that interest has not waned, with production of the 13,14-seco steroids such as 17-hydroxy-13,14-seco-androst-4-en-3-one (Khripach et al. 2004) and a cyclobutane containing compound, but any activity data has not been reported yet.
This short pre´cis of some of the anabolic steroid analogues that have been prepared clearly shows that many interesting compounds exist but with very little detailed published data on their biological activity other than some animal or in vitro testing for androgen-related mode of action. Data on activities such as toxicity and mutagenicity, which are required to be well documented in animal models before a drug can even be considered for human trials, is not available. Dealers in steroids, including those steroids present in the so-called supplements, are only interested in monetary gain and are not interested in long-term effects, or the fact that little or nothing is known about the molecule they are selling. The literature gives clues to a large variety of promising candidates for clandestine use. Most of the steroids studied in the past that have not been progressed to pharma-ceutical products, have some limited information published but do not have impor-tant animal and human toxicity and side effect data published (or even had them undertaken), and so the studies are actually being conducted on the gullible/ desperate community that buys products containing designer steroids. Thus, the only output of information on effects may appear in bodybuilding chat rooms and then in only barely understandable terms. Data for many compounds relating to oestrogenic, anti-oestrogenic or even anti-anabolic effects is not known, so pro-blems such as those currently facing the older doped East German athletes will undoubtedly occur. This assumes that these modifications to the steroid molecule do not introduce an acutely toxic or life-threatening effect. in which case the data will be in the obituary columns. The athletes that take them may or may not be aware of these issues and they use them because they believe that they are not detectable.
The making of Superdrol
 Over the course of a nearly 12-year career, Sports supplement creator Matt Cahill has continued to launch new and risky products, flourishing in the $30 billion dietary supplement industry as federal regulators struggled to keep up with his changing series of companies. Some who took his steroid suffered liver damage while others who consumed the weight-loss pills ingested a chemical (DNP) that had been banned for human use in the 1930s after users went blind. The new steroid Craze, to Cahill's knowledge, had never before been tested on humans until he and a few friends tried it themselves for a few weeks before putting it on sale in 2004.
Over the course of a nearly 12-year career, Sports supplement creator Matt Cahill has continued to launch new and risky products, flourishing in the $30 billion dietary supplement industry as federal regulators struggled to keep up with his changing series of companies. Some who took his steroid suffered liver damage while others who consumed the weight-loss pills ingested a chemical (DNP) that had been banned for human use in the 1930s after users went blind. The new steroid Craze, to Cahill's knowledge, had never before been tested on humans until he and a few friends tried it themselves for a few weeks before putting it on sale in 2004.
Even with no college degree or formal training in chemistry and despite not having a staff of scientists, getting a blockbuster designer steroid on the market was relatively easy for Cahill: "I found the chemical name in a book that contains a bunch of other steroids," he testified in the 2008 deposition. The book was Androgens and Anabolic Agents, a chemistry reference published in 1969.
Cahill said he used the Internet to find a company in China and paid about $20,000 for a kilo of the powdered compound. "I went on Alibaba.net," Cahill said. He found other firms to encapsulate the powder and put the pills into bottles, and still another to make labels for it. "I think it was LabelsForLess.com," he told attorneys.
 Cahill conceded in the 2008 deposition that the steroid that he called Methasteron and put in bottles of Superdrol had never previously been used for human consumption. But after reading studies of other, similar compounds, he concluded: "In low doses, in short periods of time, it was relatively safe." Cahill said he felt qualified to make that determination: "I had a scientific background in school, I just don't have a degree."
Cahill conceded in the 2008 deposition that the steroid that he called Methasteron and put in bottles of Superdrol had never previously been used for human consumption. But after reading studies of other, similar compounds, he concluded: "In low doses, in short periods of time, it was relatively safe." Cahill said he felt qualified to make that determination: "I had a scientific background in school, I just don't have a degree."
To determine the suggested dosage for Superdrol, Cahill said he tested the steroid on himself, had his blood tested and offered it to some friends to try. Cahill produced 2,200 bottles of Superdrol that he sold over the Internet, according to his deposition testimony. The first batch was released in late 2004, he said — the other, around early January 2005. Both sold out in less than 15 minutes, he testified. Cahill's lawyer, Aaron Goldsmith, said that Designer Supplements voluntarily stopped selling Superdrol in 2004, "prior to any reports of severe adverse reactions."
Instead, according to his deposition testimony, Cahill worked out a licensing agreement in early 2005 to let another company sell Superdrol in return for a one-time payment and royalties. The agreement with Anabolic Resources was signed in April 2005, a month after the judgment was entered in Cahill's DNP criminal case and as his prison sentence was about to start, federal court records show. In his deposition, Cahill denied that his looming incarceration had anything to do with the licensing agreement. He said the deal was to head off Anabolic Resources' plans to sell its own version of Superdrol.
Kevin Smith, who at the time was president of Anabolic Resources, told USA TODAY his company had been looking to make its own Superdrol, but that they were relying on Cahill's scientific expertise and marketing claims that the product was safe and legal. Cahill was a frequent poster in Internet bodybuilding forums, and users perceived him as "this really up-and-coming guy that makes amazing products," Smith said, adding that he and others in his company had backgrounds in marketing and sales, not science. "We told them we can take Superdrol big, and we did."
At the time of the Superdrol deal, Smith said Cahill talked about how he'd be going away for a while on a research trip. Prison was never mentioned. "He said he had to go overseas and work in different areas to find unique ingredients and botanicals. … We believed every word of it." Cahill made a similar announcement on his Designer Supplements website, writing that "in order for me to continue my passion of finding, synthesizing and creating new, cutting edge products, I need to work less on the daily operations of the business and spend more time researching and traveling to various suppliers and chem houses," according to a page captured on April 3, 2005, by the Internet Archive.
Anabolic Resources paid Cahill about $182,000 during the course of the Superdrol licensing agreement, Smith said. While Cahill was away, Smith said a potential investor flagged Superdrol as a likely illegal steroid just before the FDA sent the company a warning letter in March 2006, prompting the company to recall Superdrol from the market. "That's when we started getting calls of liver problems," Smith said. "When you get reports of people being injured from your products, there's no worse feeling."
Jareem Gunter was among the Superdrol users who sued Designer Supplements and Anabolic Resources. Gunter was a senior at Lincoln University in Jefferson City, Mo., in 2005 on a full baseball scholarship with dreams of playing professionally. Like many athletes, he was looking for an all-natural dietary supplement that would give him an edge in his workouts — but not break any rules.
After a lot of research, Gunter bought Superdrol. Within weeks, his liver was failing and he said doctors told him he needed a transplant or he could die. Gunter eventually recovered only to learn the NCAA had banned him from competition for steroid use.
- Login to post comments


