A case of toxicity from DMAA party pills in New Zealand
A case of toxicity from DMAA party pills in New Zealand
This is an incident but since we post a lot about DMAA I thought to let you know
‘Party pills’ are synthetic stimulants that emerged in New Zealand in the early 2000s. Most were 1-benzylpiperazine (BZP)-based and were widely consumed. Evidence accumulated of risk and toxicity and BZP was subsequently scheduled.1,3
Dimethylamylamine (DMAA) is one of the next generation of ‘BZP-free’ party pills.
This report describes a case of a serious complication associated with the recreational use of DMAA.
Case report
 A 21-year-old man was out with friends and purchased a quantity of legal party pills identified as ’99.9%-pure DMAA’. He took the recommended dose of 2 tablets at approximately 11:30 pm along with a capsule identified as 150 mg of caffeine. He had already ingested 1 can of beer.
A 21-year-old man was out with friends and purchased a quantity of legal party pills identified as ’99.9%-pure DMAA’. He took the recommended dose of 2 tablets at approximately 11:30 pm along with a capsule identified as 150 mg of caffeine. He had already ingested 1 can of beer.
Within 30 minutes he developed a severe global headache and called for a friend to take him home. He subsequently became confused, incontinent of urine and vomited for 2–3 hours before falling asleep. The next morning he was drowsy and had slurred speech. He did not improve during the day so at 6 pm he was taken to the local hospital emergency department (ED).
Figure 1. DMAA capsules and packaging (brand name obscured)
On arrival in the ED he was confused and had slurred speech. He was disorientated in time but not person or place. He could not give a coherent history. He had a right facial droop and right-sided weakness. There was no sympathomimetic toxicity evident, however 19 hours had elapsed since ingestion. His heart rate was 65 bpm and blood pressure was 126/66 mmHg.
An urgent computed tomograph (CT) of the brain was requested and showed a large haemorrhage (66 mm × 21 mm × 31 mm) in the region of the left basal ganglia with mass effect causing 5 mm of midline shift.
A detailed examination revealed receptive and expressive dysphasia. His short term memory was impaired. He displayed constructional and dressing dyspraxias. He had impaired stereognosis. He had right-sided weakness grade 4+/5 in upper and lowerlimbs. He suffered a focal seizure involving his right arm and was commenced on phenytoin.
A subsequent cerebral angiogram failed to show evidence of aneurysm, arteriovenous malformation or any cerebral vasculitis to account for the haemorrhage. After 5 days he showed improvement and was transferred to a brain injury rehabilitation facility.
He was discharged after 15 days of rehabilitation.
A detailed multidisciplinary assessment identified severe impairment to memory and abstract reasoning. Mild impairments of speech and right hand coordination were also noted. Identical pills obtained from the retailer were analysed and confirmed the contents as powdered 1,3 dimethylamylamine 278 mg per capsule. No caffeine, amphetamine, BZP or other stimulants were present in the sample.
Discussion
This is the first serious complication of DMAA reported in the medical literature.
DMAA is marketed as a ‘BZP-free’ party pill. BZP was initially promoted as a safe herbal alternative to illicit street drugs. It was not scheduled or controlled by drug or food safety legislation. Concerns were expressed that these designer stimulants had toxic potential.1,2 There were no studies to attest to their safety for human consumption.
Subsequent reports and research linked BZP to toxic seizures, renal impairment and multi-organ failure.3,4 BZP was banned in NZ and has also been scheduled in the United Kingdom and the European Union.
DMAA has been depicted as a benign herbal derivative (an extract of geranium oil— hence the alternate name ‘geranamine’). It is present at a concentration of less than 0.7% in naturally extracted geranium oil but the actual product is chemically synthesised and pure.
DMAA was patented by Eli Lilly in 1944 as a nasal delivery decongestant called ‘Forthane’.5 Animal studies confirmed its sympathomimetic properties and established an LD50 in rodents.6,7 Despite being sold and used in an inhaler form there is little published research on its effects in humans and none via the ingested or intravenous route.
Figure 2. CT scan of the brain showing intracerebral haemorrhage
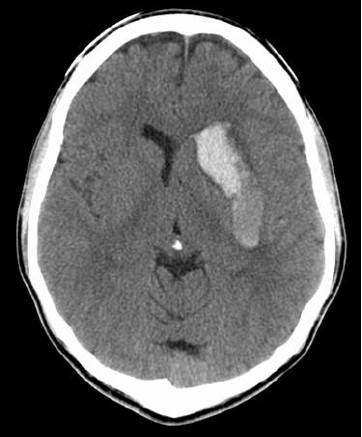 In this case, cerebral haemorrhage is likely to be linked to the ingestion of DMAA and caffeine. The time sequence is suggestive of cause and effect. Cerebral haemorrhage is associated with both episodic and chronic stimulant use. Animal and autopsy studies show that amphetamines can induce microvascular injury and angiitis.8 Druginduced hypertension may be a significant factor or may unmask underlying arteriovenous malformations or cerebral aneurysms.9,10 In this case, no angiographic evidence of vascular anomaly was found. The background rate of intracerebral haemorrhage is ‘rare’ in people under 45 years old (less than 1.9 per 100,000 person years)11 and this figure is made up mostly of cases with underlying arteriovenous malformation and aneurysm. This makes haemorrhage without underlying pathology exceedingly rare. In addition a 2009 news report cited a New Zealand Ministry of Health document detailing three cases of severe headache with vomiting and one case of cerebral haemorrhage associated with DMAA use.12 DMAA has been legally available internationally as a bodybuilding supplement but is now being sold as a legal stimulant. DMAA has been banned by World Anti-Doping Agency in their 2010 Prohibited list (listed under its synonym ‘methylhexaneamine’).13
In this case, cerebral haemorrhage is likely to be linked to the ingestion of DMAA and caffeine. The time sequence is suggestive of cause and effect. Cerebral haemorrhage is associated with both episodic and chronic stimulant use. Animal and autopsy studies show that amphetamines can induce microvascular injury and angiitis.8 Druginduced hypertension may be a significant factor or may unmask underlying arteriovenous malformations or cerebral aneurysms.9,10 In this case, no angiographic evidence of vascular anomaly was found. The background rate of intracerebral haemorrhage is ‘rare’ in people under 45 years old (less than 1.9 per 100,000 person years)11 and this figure is made up mostly of cases with underlying arteriovenous malformation and aneurysm. This makes haemorrhage without underlying pathology exceedingly rare. In addition a 2009 news report cited a New Zealand Ministry of Health document detailing three cases of severe headache with vomiting and one case of cerebral haemorrhage associated with DMAA use.12 DMAA has been legally available internationally as a bodybuilding supplement but is now being sold as a legal stimulant. DMAA has been banned by World Anti-Doping Agency in their 2010 Prohibited list (listed under its synonym ‘methylhexaneamine’).13
The New Zealand Ministry of Health has moved to regulate the sale of DMAA to those 18 years or older, but it is still legally available for human consumption.
References:
1. Gee P; Richardson S; Woltersdorf W; Moore G. Toxic effects of BZP-based herbal party pills
in humans: a prospective study N Z Med J2005 Dec 16;118(1227).
2. D. Wood, P. Dargan, J. Button, D. Holt, H. Ovaska, J. Ramsey, A. Jones Collapse, reported
seizure—and an unexpected pill The Lancet, 2007;369(9571):1490-1490
3. Gee P, Gilbert M, Richardson R, et al. Toxicity from therecreational use of 1-benzylpiperazine
Clin Tox 2008;46(9):802-807 (doi: 10.1080/15563650802307602).
4. Alansari M, Hamilton D Nephrotoxicity of BZP-based herbal party pills: a New Zealand case
report N Z Med J 2006 May 5;119(1233).
5. Council on Pharmacy and Chemistry Methylhexamine Forthane (Lilly). JAMA.
1950;143:1155-1157.
6. Ellis S The effect of amines on the blood sugar of the rat. J Pharmacol Exp Ther.
1951;101:92-100.
7. Miya TS, Edwards I. A pharmacological study of certain alkoxyalkylamines J Pharm Sci.
1953;42(2):107-10.
8. Olsen ER. Intracranial hemorrhage and amphetamine usage. Review of the effects of
amphetamines on the central nervous system. Angiology. 1977;28(7):464-71.
9. Buxton N, McConachie NS. Amphetamine abuse and intracranial haemorrhage. J R Soc Med.
2000 Sep;93(9):472-7.
10. McEvoy AW, Kitchen ND, Thomas DGT Intracerebral haemorrhage in young adults: the
emerging importance of drug misuse BMJ 2000;320(7245):1322
(doi:10.1136/bmj.320.7245.1322).
11. van Asch, CJ, Luitse, MJ, Rinkel, GJ, et al. Incidence, case fatality, and functional outcome of
intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic
review and meta-analysis. Lancet Neurol 2010;9:167.
12. Chalmers A. Officials seek limit on toxic pill ingredient. The Dominion Post, 12 January 2009
edition. 13. World Anti Doping Agency. The World Anti-Doping Code. The 2010 Prohibited List.
International standard.
14. Press Release: New Zealand Government. Saturday 7 November 2009.
- Login to post comments


