Aromasin (and other aromatase inhibitors)
Aromasin (and other aromatase inhibitors)
 Doctors give anti-estrogens in cancer patients with tumors that grow faster by estradiol.
Doctors give anti-estrogens in cancer patients with tumors that grow faster by estradiol.
Chemical athletes use anti-estrogens because they want to prevent their synthetic hormones to convert into estradiol, with all its unpleasant side effects. The side effects that users of anti-estrogens experience, are an increased risk of bone fractures and pain in muscles and joints, are due to the drop in estrogen levels. This increases after years of use. The anti-estrogen on itself are only toxic in idioticly high doses.
The anti-estrogen Exemestane is not suitable to restore the natural production of testosterone after a steroids cycle. According to a molecular study of U.S. cancer researchers (13).
Exemestane is again very interesting to use during an anabolic cycle. Exemestane, which also marketed as Aromasin, Arimidex and Letrozole is like an anti-estrogen in the third generation. Exemestane inhibits the enzyme aromatase, which converts testosterone and androstenedione into the female hormone estradiol.
According to the textbooks exemestane is a suicide inhibitor of aromatase. This means that it destroys the enzyme forever. Only when the body has created new enzymes the conversion of testosterone to estradiol is possible again. Arimidex and Letrozole in contrast only temporarily shut down the action of aromatase. They are called reversible inhibitors. Later discussed as type 1 and 2.
Aromasin (Exemestane) is a third generation Aromatase Inhibitor (AI) just like Arimidex (Anastrozole) and Femera (Letrozole). Both of those two drugs are very efficient at stopping the conversion of androgens into estrogen, and since we have them, why bother with Aromasin? It’s a little harder to get than the other two commonly used aromatase inhibitors, because it’s not in high demand, and there’s never been a readily apparent advantage to using it. And I mean…lets face it: It’s awkward-sounding. Aromasin doesn’t have much of a ring to it, and exemestane is even worse. Arimidex has a bunch of cool abbreviations ("A-dex" or just ‘dex) and even Letrozole is just "Letro" to most people. Where’s the cool nickname for
Aromasin/exemestane? A-Sin? E-Stane? It just doesn’t work. It’s the black sheep of AIs. And why do we even need it when we have Letrozole, which is by far the most efficient AI for stopping aromatization (the process by which your body converts testosterone into estrogen)? Letro can reduce estrogen levels by 98% or greater; clinically a dose as low as 100mcgs has been shown to provide maximum aromatase inhibition (2)!
So why would we need any other AIs? Well, first of all, estrogen is necessary for healthy joints (3) as well as a healthy immune system (4). So getting rid of 98% of the estrogen in your body for an extended period of time may not be the best of ideas. This may be useful on an extreme cutting cycle, leading up to a bodybuilding contest, or if you are particularly prone to gyno, but certainly can’t be used safely for extended periods of time without compromising your joints and immune system.
That leaves us with Arimidex, which isn’t as potent as Letrozole, but at .5mgs/day will still get rid of around half (50%) of the estrogen in your body. Problem solved, right? Use Arimidex on your typical cycles, and if you are very prone to gyno or are getting ready for a contest, use Letro. Letroze inhibits the highest percentage of estrogen and makes you extremely dry!! But it also wrecks the sex drive (libido) of many users and for a long time, some users said it took them 3 month to recover. You should use letroze during your whole cycle, because it will get rid of estradiol, which you need to keep the elasticity in your bones. For this reason women with breastcancer have higher chances on fractures and osteoporosis while using letroze. Nolvadex works in the opposite way it adds strength to your bones because it is a bone agonist.
You should never use an aromatase inhibitor (AI )alone, its better to use a SERM (Nolvadex) with an AI to offset the decreased HDL caused by the AIs. Nolvadex also reduces the estrogenic activity in the breasttissue. Also remember that AI’s can’t reduce the side-effects of Nandrolone (Deca Durabolin), you need Nolvadex for that. Nolvadex acts agonistically on lipid profiles in your blood, thus running tamoxifen with your AI’s will improve your cholesterol profile (VDL/LDL –HDL balance) even if you’re not on a cycle. Also keep in mind that anbolic androgenic steroids mostly have a bad influence on your bloodlipid balance. Letroze plasma concentrations where lower when letroze as given in combination with tamoxifen with a mean reduction of 37,6% (16)Clin Cancer Res September 1999 Clin Cancer Res September 1999
But what about Post Cycle Therapy (PCT)?
I think at this point most people are sold on the use of Nolvadex (Tamoxifen Citrate) instead of Clomid for post cycle therapy (PCT), since both compete estrogen at the receptor site, both increase serum test levels, and both drugs may also alter blood lipid profiles favorably (6). But since 20mgs of Tamoxifen is equal to 150mgs of clomid for purposes of testosterone elevation, FSH and LH, but Tamoxifen doesn’t decrease the LH response to LHRH (6) I think most people agree to Nolvadex’s superiority for PCT.
Aromasin with Nolvadex
I’ve always been in favor of using Nolvadex during PCT, along with an AI, because reducing estrogen levels has been positively correlated with an increase in testosterone (7) so in my mind, it’s be beneficial to increase testosterone by as many mechanisms as possible while trying to recover your endogenous testosterone levels after a cycle. SO which AI do we use? Letro or A-dex? Well, why don’t we just keep using whichever one we used during the cycle, and add in some Nolvadex? Unfortunately, Nolvadex will significantly reduce the blood plasma levels of both Letrozole as well as Arimidex (8). So if we choose to use one of them with our Nolvadex on PCT, we’re throwing away a bit of money as the Nolvadex will be reducing their effectiveness.
This, of course, is where Aromasin comes in, at 20-25mgs/day.
Aromasin, at that dose, will raise your testosterone levels by about 60%, and also help out your free to bound testosterone ratio by lowering levels of Sex Hormone Binding Globulin (SHBG), by about 20% (12)…SHBG is that nasty enzyme that binds to testosterone and renders it useless for building muscle. But what about using it along with Nolvadex for PCT?
Difference Between Type-I and Type-II Aromatase Inhibitors
To understand why Aromasin may be useful in conjunction with Nolvadex while both Letro and A-dex suffer reduced effectiveness, we’ll need to first understand the differences between a Type-I and Type-II Aromatase Inhibitor. Type I inhibitors (like Aromasin) are actually steroidal compounds, while type II inhibitors (like Letro and A-dex) are non-steroidal drugs. Hence, androgenic side effects are very possible with Type-I AIs, and they should probably be avoided by women. Of course, there are some similarities between the two types of AIs…both type I & type II AIs mimic normal substrates (essentially androgens), allowing them to compete with the substrate for access to the binding site on the aromatase enzyme. After this binding, the next step is where things differ greatly for the two different types of AI’s. In the case of a type-I AI, the noncompetitive inhibitor will bind, and the enzyme initiates a sequence of hydroxylation; this hydroxylation produces an unbreakable covalent bond between the inhibitor and the enzyme protein. Now, enzyme activity is permanently blocked; even if all unattached inhibitor is removed. Aromatase enzyme activity can only be restored by new enzyme synthesis. Now, on the other hand, competitive inhibitors, called type II AI’s, reversibly bind to the active enzyme site, and one of two things can happen: 1.) either no enzyme activity is triggered or 2.) the enzyme is somehow triggered without effect. The type II inhibitor can now actually disassociate from the binding site, eventually allowing renewed competition between the inhibitor and the substrate for binding to the site. This means that the effectiveness of competitive aromatase inhibitors depends on the relative concentrations and affinities of both the inhibitor and the substrate, while this is not so for noncompetitive inhibitors. Aromasin is a type-I inhibitor, meaning that once it has done its job, and deactivated the aromatase enzyme, we don’t need it anymore. Letrozole and Arimidex actually need to remain present to continue their effects. This is possibly why Nolvadex does not alter the pharmacokinetics of Aromasin (11).
Since Aromasin is about 65% efficient at suppressing estrogen (10), it’s certainly a very powerful agent, especially considering you won’t experience reduced effectiveness because of your concurrent use of Nolvadex or from any sort of tolerance developed by using other AIs on your cycle(9). There is also a decent amount of preclinical data suggesting that Aromasin has a beneficial effect on bone mineral metabolism that is not seen with non-steroidal agents, and it may also have beneficial effects on lipid metabolism that are not found in the non-steroidal Letro and A-dex (9).
Anti-estrogen Exemestane has androgenic effects
This also applies to exemestane. The substance can be described as a boldenone precursor with a methylene group at C6. This molecule does nothing with steroidreceptors. But in the eighties when the first experiments with exemestane where performed, chemists discovered that enzymes in the body convert exemestane into compounds that indeed have an hormonal effect (13).
 The scientists have investigated such an active exemestane-metabolite. He's created when exemestane carbonyl on C17 turns into a hydroxyl group. The structure at left is that of exemestane, on the right you see the 17-hydroxy metabolite.
The scientists have investigated such an active exemestane-metabolite. He's created when exemestane carbonyl on C17 turns into a hydroxyl group. The structure at left is that of exemestane, on the right you see the 17-hydroxy metabolite.
Androgens are not very active as they have a carbonyl in C17 down. Turns it into a carbonyl hydroxyl group, then that androgens generally more active pieces. The scietists wondered whether that also applies to exemestane. In the body of users find that 17-hydroxy metabolite at concentrations one tenth of the average concentration of exemestane itself. That's a lot.
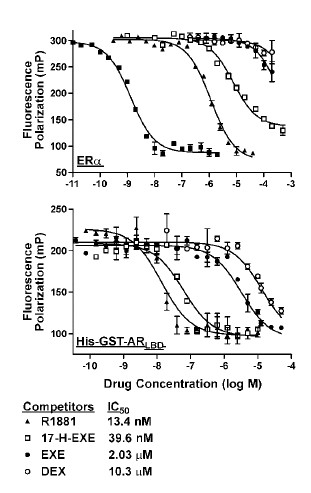 Long story short: yes, 17-hydroxy-Exemestane is a pretty strong androgen. Below you can see how well exemestane [EXE] and the 17-hydroxy metabolite attach to estrogen [first graph] and the androgen receptor [second graph]. E2 stands for estradiol, DEX for dexamethasone, R1881 for the super-androgen methyltrenbolone (aka metribolone).
Long story short: yes, 17-hydroxy-Exemestane is a pretty strong androgen. Below you can see how well exemestane [EXE] and the 17-hydroxy metabolite attach to estrogen [first graph] and the androgen receptor [second graph]. E2 stands for estradiol, DEX for dexamethasone, R1881 for the super-androgen methyltrenbolone (aka metribolone).
The further the curve of a substance is situated to the right, the weaker that substance attaches to the receptor. Exemestane and its metabolite are hardly estrogen, but the 17-hydroxy metabolite interacts, even in low concentrations, with the androgen receptor. Computer simulations, in which the metabolite attached itself to the androgen receptor, confirmed that outcome.
The scientits also found that 17-hydroxy-Exemestane promotes the growth of cancer cells in a test tube. Which confirms its androgenity.
For physicians that is bad news. If they have a patient with an androgen-sensitive cancern, and give that patient exemestane, they also have to provide an anti-androgen. Chemical athletes find that exemestane is not suitable to use during the post-cycle (PCT). Anti-estrogens accelerate the production of testosterone, but androgens inhibit this process.
On the contrary, as part of a cycle exemestane seems much more interesting. Perhaps athletes with modest chemical demands may even wish to grow on exemestane alone: the drug increases the levels of testosterone, lowers estradiol levels and even converts into a compound with an anabolic / androgenic activity.
The above would to some athletes even apply in duplication, according to the article. "Furthermore, a subpopulation of patients may exist who metabolize exemestane at higher rates, leading to correspondingly higher circulating 17-hydroexemestane levels", the researchers write. "For instance, one of three patients administered 800 mg of exemestane, the highest dose evaluated, achieved 17-hydroexemestane plasma levels approximately one-half the level of the parent compound."
An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane (14).
BACKGROUND: The newer generation, nonsteroidal aromatase inhibitors (AIs) anastrozole and letrozole have shown superior efficacy compared with tamoxifen as first-line treatments and compared with megestrol acetate as second-line therapy in postmenopausal women with advanced breast carcinoma. In an open-label, Phase II trial, it was reported that exemestane showed numerical superiority compared with tamoxifen for objective response and clinical benefit. Because these agents ultimately may be administered for periods of up to 5 years in the adjuvant setting, it is of increasing importance to assess their tolerability and pharmacologic profiles. METHODS: In the absence of data from direct clinical comparisons, the published literature was reviewed for the clinical pharmacology, pharmacokinetic characteristics, and selectivity profiles of anastrozole, letrozole, and exemestane. RESULTS: At clinically administered doses, the plasma half-lives of anastrozole (1 mg once daily), letrozole (2.5 mg once daily), and exemestane (25 mg once daily) were 41-48 hours, 2-4 days, and 27 hours, respectively. The time to steady-state plasma levels was 7 days for both anastrozole and exemestane and 60 days for letrozole. Androgenic side effects have been reported only with exemestane. Anastrozole treatment had no impact on plasma lipid levels, whereas both letrozole and exemestane had an unfavorable effect on plasma lipid levels. In indirect comparisons, anastrozole showed the highest degree of selectivity compared with letrozole and exemestane in terms of a lack of effect on adrenosteroidogenesis. CONCLUSIONS: All three AIs demonstrated clinical efficacy over preexisting treatments. However, there were differences in terms of pharmacokinetics and effects on lipid levels and adrenosteroidogenesis. The long-term clinical significance of these differences remains to be elucidated.
Pharmacokinetics and Dose Finding of a Potent Aromatase Inhibitor, Aromasin (Exemestane), in Young Males (15)
Suppression of estrogen, via estrogen receptor or aromatase blockade, is being investigated in the treatment of different conditions. Exemestane (Aromasin) is a potent and selective irreversible aromatase inhibitor. To characterize its suppression of estrogen and its pharmacokinetic (PK) properties in males, healthy eugonadal subjects (14–26 yr of age) were recruited. In a cross-over study, 12 were randomly assigned to 25 and 50 mg exemestane daily, orally, for 10 d with a 14-d washout period. Blood was withdrawn before and 24 h after the last dose of each treatment period. A PK study was performed (n = 10) using a 25-mg dose. Exemestane suppressed plasma estradiol comparably with either dose [25 mg, 38% (P 0.002); 50 mg, 32% (P 0.008)], with a reciprocal increase in testosterone concentrations (60% and 56%; P 0.003 for both). Plasma lipids and IGF-I concentrations were unaffected by treatment. The PK properties of the 25-mg dose showed the highest exemestane concentrations 1 h after administration, indicating rapid absorption. The terminal half-life was 8.9 h. Maximal estradiol suppression of 62 ± 14% was observed at 12 h. The drug was well tolerated. In conclusion, exemestane is a potent aromatase inhibitor in men and an alternative to the choice of available inhibitors. Long-term efficacy and safety will need further study.
Proviron
Proviron has four distinct uses in the world of bodybuilding. The first being the result of its structure. It is 5-alpha reduced and not capable of forming estrogen, yet it nonetheless has a much higher affinity for the aromatase enzyme (which converts testosterone to estrogen) than testosterone does. That means in administering it with testosterone or another aromatizable compound, it prevents estrogen build-up because it binds to the aromatase enzyme very strongly, thereby preventing these steroids from interacting with it and forming estrogen. So Mesterolone use has the extreme benefit of reducing estrogenic side-effects and water retention noted with other steroids, and as such still help to provide mostly lean gains. Its also been suggested that it may actually downgrade the actual estrogen receptor making it doubly effective at reducing circulating estrogen levels.
The second use is in enhancing the potency of testosterone. Testosterone in the body at normal physiological levels is mostly inactive. As much as 97 or 98 percent of testosterone in that amount is bound to sex hormone binding globulin (SHBG) and albumin, two proteins. In such a form testosterone is mostly inactive. But as with the aromatase enzyme, DHT has a higher affinity for these proteins than testosterone does, so when administered simultaneously the mesterolone will attach to the SHBG and albumin, leaving larger amounts of free testosterone to mediate anabolic activities such as protein synthesis. Another way in which it helps to increase gains. Its also another part of the equation that makes it ineffective on its own, as binding to these proteins too, would render it a non-issue at the androgen receptor.
Thirdly, mesterolone is added in pre-contest phases to increase a distinct hardness and muscle density. Probably due to its reduction in circulating estrogen, perhaps due to the downregulating of the estrogen receptor in muscle tissue, it decreases the total water build-up of the body giving its user a much leaner look, and a visual effect of possessing "harder" muscles with more cuts and striations. Proviron is often used as a last-minute secret by a lot of bodybuilders and both actors and models have used it time and again to deliver top shape day in day out, when needed. Like the other methylated DHT compound, drostanolone, mesterolone is particularly potent in achieving this feat.
Lastly Proviron is used during a cycle of certain hormones such as nandrolone, with a distinct lack of androgenic nature, or perhaps 5-alpha reduced hormones that don't have the same affinities as DHT does. Such compounds, thinking of trenbolone, nandrolone and such in particular, have been known to decrease libido. Limiting the athlete to perform sexually being the logical result. DHT plays a key role in this process and is therefore administered in conjunction with such steroids to ease or relieve this annoying side-effect. Proviron is also commonly prescribed by doctors to people with low levels of testosterone, or patients with chronic impotence. Its not perceived as a powerful anabolic, but it gets the job done equally well if not better than other anabolic steroids making it a favourite in medical practices due to its lower chance of abuse. (..binding SHBG..)
References:
- Clin Cancer Res. 2005 Apr 15;11(8):2809-21.
- 2. J Clin Endocrinol Metab. 1995 Sep;80(9):2658-60.
- [Clinical aspects of estrogen and bone metabolism] Clin Calcium. 2002 Sep;12(9):1246-51. Japanese.
- Science, Vol 283, Issue 5406, 1277-1278 , 26 February 1999
- J Clin Endocrinol Metab 2000 Jul;85(7):2370-7, "Estrogen Suppression in Males"
- Fertil Steril. 1978 Mar;29(3):320-7
- J Clin Endocrinol Metab. 2004 Mar;89(3):1174-80
- .J Steroid Biochem Mol Biol. 2001 Dec;79(1-5):85-91.
- The Oncologist, Vol. 9, No. 2, 126–136, April 2004
- Endocrinological and clinical evaluation of exemestane, a new steroidal aromatase inhibitor. Br. J. Cancer, 72: 1007-1012, 1995
- Clinical Cancer Research Vol. 10, 1943-1948, March 2004
- The Journal of Clinical Endocrinology & Metabolism Vol. 88, No. 12 5951-5956
- Antiestrogen Exemestane has androgenic effects Molecular Cancer Therapeutics November 2007, 6 (11) :2817-27.
- Clin Cancer Res. 2003 Jan 9Pharmacology and pharmacokinetics of the newer generation aromatase inhibitors.
- JCEM December 1, 2003 vol. 88 Pharmacokinetics and Dose Finding of a Potent Aromatase Inhibitor, Aromasin (Exemestane), in Young Males
- Clin Cancer Res 1999 September 5 Impact of Tamoxifen on the Pharmacokinetics and Endocrine Effects of the Aromatase Inhibitor Letrozole
- Login to post comments


