Analogues of THG

Most of us know that generic and underground drugs often contain active ingredients that are not listed on the label. Just like tainted herbal or nutritional supplements.
One of the best examples in this case are drugs/supplements to fight erectile dysfunction. Over 45 different versions of drugs in the same class of Viagra have now been found in male sexual supplements. A Dutch study found that 75% of the products sold in the Netherlands contained at least one analogue, or chemical variant that has the same effect as Viagra.
“ We’re talking about a massive number of brands and millions of millions of pills that are tainted,” Pieter Cohen, assistant professor of medicine at Harvard Medical School, says. “Hundreds of millions of pills are being produced every month that are spiked with these prescription medications — and worse, entirely novel drugs.” “The presence of the new medications is particularly concerning since there are no data to support their safety, much less their efficacy.” Ahum, if these pills are ineffective you’ll find out soon, right?
We’re talking about a massive number of brands and millions of millions of pills that are tainted,” Pieter Cohen, assistant professor of medicine at Harvard Medical School, says. “Hundreds of millions of pills are being produced every month that are spiked with these prescription medications — and worse, entirely novel drugs.” “The presence of the new medications is particularly concerning since there are no data to support their safety, much less their efficacy.” Ahum, if these pills are ineffective you’ll find out soon, right?
A lesser known fact is that also anabolic steroids contain analogues that are not the ones you thought you acquired. I want to share some analytical results about THG (tetrahydro-gestrinone)later in this blogpost, but first what again is THG?
Patrick Arnold is a chemistry graduate and also a bodybuilder. He dug deep into the chemical literature, searching for steroids that had been tested but never brought to market, so-called ‘designer steroids’.
 He became involved with the Bay Area Laboratory Co-Operative (BALCO), who supplied these performance enhancing drugs to athletes. Norbolethone was one of these, it had never been marketed, so it was not a steroid that testers were expecting. When the testers did detect that, Arnold came up with an even s marter idea, a molecule that had never, ever, been made before; it was to become famous as THG.
He became involved with the Bay Area Laboratory Co-Operative (BALCO), who supplied these performance enhancing drugs to athletes. Norbolethone was one of these, it had never been marketed, so it was not a steroid that testers were expecting. When the testers did detect that, Arnold came up with an even s marter idea, a molecule that had never, ever, been made before; it was to become famous as THG.
 A steroid called gestrinone, on the other hand, was commercially available. It was originally devised, in 1974, as a contraceptive steroid. It has an alkynyl group – containing a carbon-carbon triple bond – attached at carbon 17. Arnold realised that converting the alkyne group to an alkyl group would make gestrinone into a carbon-17 alkylated steroid, likely to have real anabolic properties. It would just require the addition of four hydrogen atoms to turn the gestrinone molecule into tetrahydrogestrinone. To a skilled organic chemist like Arnold, this was easy, it just needed the reaction of hydrogen gas in the presence of a catalyst, and it was turned into THG. Once this had been done, THG was ready to be used. It is the presence of the alkyl group at carbon-17 that enables it to make stronger van der Waals' contacts with the human androgenic receptor than do the other steroids, and that is why it has such strong androgenic properties, as well as its anabolic effects.
A steroid called gestrinone, on the other hand, was commercially available. It was originally devised, in 1974, as a contraceptive steroid. It has an alkynyl group – containing a carbon-carbon triple bond – attached at carbon 17. Arnold realised that converting the alkyne group to an alkyl group would make gestrinone into a carbon-17 alkylated steroid, likely to have real anabolic properties. It would just require the addition of four hydrogen atoms to turn the gestrinone molecule into tetrahydrogestrinone. To a skilled organic chemist like Arnold, this was easy, it just needed the reaction of hydrogen gas in the presence of a catalyst, and it was turned into THG. Once this had been done, THG was ready to be used. It is the presence of the alkyl group at carbon-17 that enables it to make stronger van der Waals' contacts with the human androgenic receptor than do the other steroids, and that is why it has such strong androgenic properties, as well as its anabolic effects.
No one knows how many athletes took THG, or indeed how long its use would have gone on for had it not been for a snitch. Early in June 2003, an athletics coach contacted an official of the United States Anti-Doping Agency, saying that the head of BALCO had been supplying leading athletes with drugs; the coach promised to send the USADA a used syringe that had been thrown away at an athletics meeting.
THG is a highly potent agonist for the androgen and progesterone receptors, around 10 times more potent than the comparison drugs nandrolone or trenbolone, but with no estrogenic activity. It has been found to bind to the androgen receptor with similar affinity to dihydrotestosterone and produces growth of muscle tissue. According to Patrick Arnold, due to the drug's potency, he never had to supply significant quantities to BALCO, because "just a couple of drops under the tongue" were a sufficient dose.
When THG reaches the nucleus of a cell, it binds to the androgen receptor at the ligand-binding pocket. Here it changes the expression of a variety of genes, turning on several anabolic and androgenic functions.
I
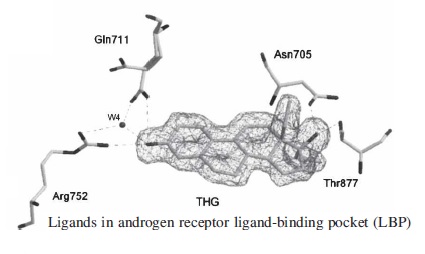 t is the ligand’s structure which determines the number of interactions that can take place with the human androgen receptor ligand-binding domain. Even minor modifications in the ligand's structure have a great impact on the strength of the interactions this ligand has with the androgen receptor. THG, possessing a high affinity, establishes more van der Waals contacts with the receptor than with many other steroids. It is this higher affinity and specific geometry of THG which makes these interactions with the Androgen Receptor so strong, resulting in THG’s potency. Canadian research found that attaching extra methyl-ethyl or ethynyl groups to the steroid body increased its potency.
t is the ligand’s structure which determines the number of interactions that can take place with the human androgen receptor ligand-binding domain. Even minor modifications in the ligand's structure have a great impact on the strength of the interactions this ligand has with the androgen receptor. THG, possessing a high affinity, establishes more van der Waals contacts with the receptor than with many other steroids. It is this higher affinity and specific geometry of THG which makes these interactions with the Androgen Receptor so strong, resulting in THG’s potency. Canadian research found that attaching extra methyl-ethyl or ethynyl groups to the steroid body increased its potency.
Labrie et al. (2005) have found that R1881 (Metribolone), DHT, and testosterone have relative potencies of 72%, 58%, and 7% compared with the 100% value set for THG, the compound showing the highest affinity for AR. Having obtained the three crystal structures, we performed precise structural analysis and comparison in order to determine the way by which the hAR (human androgenic receptor) can adapt the structure of its steroid-binding site to accommodate these structurally different ligands.
B odybuilder Drug
odybuilder Drug
Tim Montgomery of the United States, the world 100 meters record holder, told the grand jury "The clear" (THG) caused him "tightness, a lot of water retention,". "My results (in races) was horrible, a lot of people's was. A lot of people ran terrible on it.”
The sprinter said he received "the clear" and human growth hormone regularly over an eight-month span as part of "Project World Record," a Conte- sponsored effort to help Montgomery set a new world mark in the 100-meter dash.
"Kelli White cramped up and do awful on it," he continued. "She was feeling the same side effects (and) Charlie Francis felt the same thing. He was like, they had another kid up there that was taking 'the clear' and he ran terrible."
Montgomery said he and Francis concluded that Conte's drug, described as a steroid that had been chemically altered so it would not show up on doping tests, "was made for bodybuilders, not for sprinters;" it was making him muscle-bound without improving his performance, Montgomery complained.
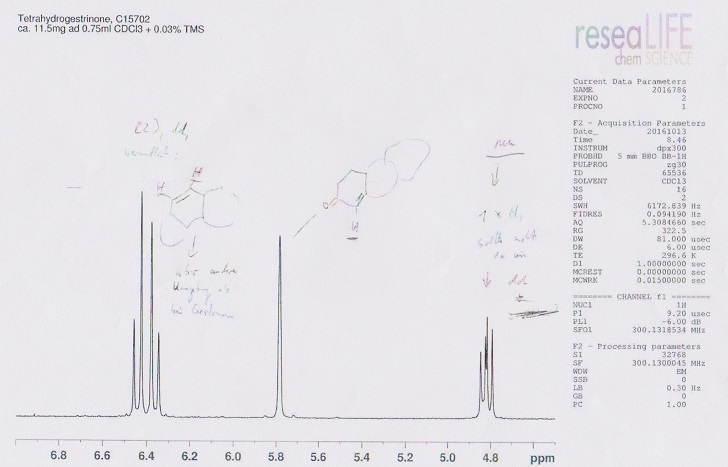

Above you see a few analyses I had performed on a sample of raw powder bought as THG.
The analyst mailed me: On the IR-spectras by 320 cm-1 you see by Gestrinon the absorption of the triple-link. By THG this absorption is missing. Therefore we can say the hydrogenation did destruct this triple. But in the field of the Fingerprint you can see the “THG” has not the structure from Gestrinon.
The NMR-spectras show Gestrinon has exactly the expected 24 protons. Theoretically THG must have 28 protons. We found 35 protons. And the structure of Gestrinon is not matching.
He concluded: There are 2 possibilities, first the laboratory over-hydrogenated and the structure from Gestrinon is changed. Or we have a mixture from THG and another product.
I really wonder how much designersteroids from designersteroids are circulating in the underground and how these products work, not as obvious as sildenafil analogues, unfortunally.
Preparation of THG
 Hydrogenation of gestrinone produced a number of products, including dihydrogestrinone, hexahydrogestrinone, and a compound tentatively identified as norbolethone. Careful controlled hydrogenation of gestrinone is absolute requirement to produce a quality product. This is how it is done:
Hydrogenation of gestrinone produced a number of products, including dihydrogestrinone, hexahydrogestrinone, and a compound tentatively identified as norbolethone. Careful controlled hydrogenation of gestrinone is absolute requirement to produce a quality product. This is how it is done:
Gestrinone (100 mg, 0.32 mMol) dissolved in 15 mL of toluene was treated with 3 mg of Lindlar catalyst (palladium, 5% on calcium carbonate poisoned with lead) Following air removal the flask was filled with hydrogen. The reaction mixture was stirred for 36 hours under hydrogen at atmospheric pressure, then filtered through celite, after which the toluene was evaporated under reduced pressure. Chromatographic purification on silica gel, with elution by hexane-tert-butyl methyl ether (2:1) yielded 82 mg of THG as a yellowish oil of 98% purity (GC-MS).



