Anabolic Oral Steroids and the Liver
Anabolic Oral Steroids and the Liver
.jpg) Liver damage is probably the most sensationalized of all possible side effects from oral steroid use. The media often focuses on this particular problem as if it occurs with every steroid, and in every person who takes them. Nothing could be further than the truth. Most anabolic steroids which are ingested orally pass through the liver, which functions as the body´s filtration system. When something goes through the liver, it is broken down by various enzymes, and passed along into the bloodstream. Most research on orally administered anabolic steroids focus on the fact that liver enzymes are elevated following ingestion. But does this necessarily mean that the liver is being damaged, does it? Of course not. All Oral Steroids put stress on the liver. So does alcohol, prescription drugs, asprin and physical conditions lile overweight/obesity.
Liver damage is probably the most sensationalized of all possible side effects from oral steroid use. The media often focuses on this particular problem as if it occurs with every steroid, and in every person who takes them. Nothing could be further than the truth. Most anabolic steroids which are ingested orally pass through the liver, which functions as the body´s filtration system. When something goes through the liver, it is broken down by various enzymes, and passed along into the bloodstream. Most research on orally administered anabolic steroids focus on the fact that liver enzymes are elevated following ingestion. But does this necessarily mean that the liver is being damaged, does it? Of course not. All Oral Steroids put stress on the liver. So does alcohol, prescription drugs, asprin and physical conditions lile overweight/obesity.
Elevated liver enzymes
Regarding elevations of SGOT, and SGPT; let's note that the liver is basically a "factory" that manufactures things like proteins and processes or destroys things like medications. There are many enzymes used in these processes and SGOT and SGPT are only two of them. However, they are two very important ones and they are the ones with which the state of the liver is generally measured.
SGPT is fairly specific to the liver, whereas SGOT is found in other organs like muscles. Therefore, it is important to note that elevated SGOT can sometimes be seen after a good workout, when the muscles have been releasing and transferring bio-chemicals. So we see that SGOT can be elevated just because of intense muscular activity.
Another thing to remember is that these enzymes will rise when almost any drug is taken by the patient. I have seen the SGPT go to two times normal when patients take four tetracycline capsules!
I would say that almost all drugs are somewhat "toxic" to the liver, in that there's a certain amount of work the liver does to remove the drugs from the system. By convention, doctors will stop medications if liver enzymes approach three times normal - just as a precaution.
Anabolic steroids are processed by the liver. C-17 alkylated oral steroids (steroids with an alkyl group added at the alpha position of the "C-17" or number 17 carbon atom of the molecule to withstand total degradation on their first pass through the liver are unusually harsh on the liver. For this reason, even moderate short-term administration of these C-17 oral steroids can effect liver function test readings. Elevated liver counts indicating liver stress (toxicity) have been reported in recent studies of somewhat moderate oral anabolic steroid therapy (daily doses of 40 and 80 mg of oxandrolone [Oxandrin, formerly Anavar]) as reported in the online periodical Medibolics, edited by Michael Mooney (www.medibolics.com). However, these elevated liver function readings will return to normal after cessation of a moderate, short-term steroid cycle. I could find not one case to the contrary. Further, it is recognized that intense weight training alone often causes changes in liver function tests, including SGOT, SGPT and LDH (this is something that all physicians monitoring athletes using anabolics should be familiar with).
The more serious liver problems attributed to anabolic steroid use include hepatocellular carcinoma (liver cancer) and peliosis hepatitis (blood-filled sacs within the liver). But the majority of cases reporting liver problems have dealt with extremely sick and elderly patients treated with C-17 alkylated oral steroids for years of continuous use, and many of these patients had a particular type of anemia linked to liver tumors even without anabolic steroid therapy.
What is common in all scientific studies is, we can only prove that any steroid, that is believed to be hepatotoxic, only increases liver activity. I’ll say it again, where is the correlation to hepatotoxicity? We know that if the liver is running at 100% for long periods this may cause complications, but this is akin to any other chemical, which is metabolized by the liver. Ever noticed that liver cancer due to alcoholism takes decades of constant alcohol abuse.
While the dangers of anabolics to athletes' livers appear to have been highly exaggerated, it must be recognized that an apparently healthy athlete with a previously existing but undiscovered liver problem could do serious damage to himself by self-administering C-17 oral anabolic steroids. For this reason alone, it would be quite irresponsible for any athlete to use anabolic steroids without having a physician regularly conduct blood tests to monitor liver function
But the most important consideration here that we should underline is that the relative "liver toxicity" of anabolic steroids that are injectable and oil-based is generally not a major consideration, because these compounds are basically metabolized the same as the natural testosterone that is already in the body. However, we may see liver enzyme increases just because the liver is working to remove these steroids, the same as it does any other foreign chemical.
Commonly, studies that focus on steroid toxicity often use absurd doses, or incorrectly focus on liver activity instead of damage. The liver functions as the filter for the human body.It´s going to be activated whenever something (not just a steroid) passes through it. Does that show that steroids damage the liver?
Alternative routes like IM or sub-Q injection avoid the first-pass effect because they allow drugs to be absorbed directly into the systemic circulation. Thus, they are much less harmful. However, some 17 alpha-alkylated steroids can be injected IM (stanozolol, methandrostenolone) and may cause problems at high doses or for prolonged periods.
Scientific Studies
A computer search of the medical literature looking for steroid-associated liver tumors could find only three in athletes (Friedl, 1990). Of the three athletes, one was using 700 mg of oxymetholone a week for five straight years, and one had a tumor more indicative of classic liver cancer than of steroid-associated tumors. Virtually all of the reported liver problems seemed to occur with the 17 alpha-alkylated oral steroids. There have been no cysts or liver tumors reported in athletes using the 17 beta-esterified injectable steroids (Wright & Cowart, ). It has been noted that injectable steroids generally appear to have little effect on the liver at all (Haupt, 1993,).
Recent studies continue to suggest that reports of serious adverse effects of anabolic steroids upon the liver in healthy athletes may be highly overstated. In a study of athletes, of the 53 current or past steroid users who underwent laboratory testing, only one subject displayed an abnormal liver test (Pope & Katz, 1994, p. 379; incidentally, on physical examination, not one user displayed evidence of any major abnormalities possibly attributable to steroids, such as high blood pressure, edema, acne or hair loss.) Another study tested one of the most powerful and reputedly dangerously toxic anabolic steroids for 30 weeks on HIV positive men and women (Hengge et al.). Oxymetholone, formerly known as Anadrol in the U.S. and a C-17 alkylated oral steroid, was administered in a dosage of over 1,000 mg per week (more than that used by many bodybuilders, and for a much longer duration of uninterrupted use). The results were significant gains in lean muscle mass -- even without any weightlifting. Even more importantly - and surprisingly -- there were no significant problems with liver function, water retention, or virilization side effects (it will be interesting to see whether further studies yield consistent findings at such high dosages).
There was an eight-week study (Molano F, 1999), which looked at the effects of an 8-week cycle of Oral steroids. The steroids examined were Halotestin (Fluoxymesterone), Dianabol (methylandrostanolone), or Winstrol (Stanozolol) on rats at the dose of 2mg/kg-body weight, administered five times a week for 8 weeks. That’s almost 200mgs/day of any of those steroids, for a 200lb user. That is, I´ll speculate, much more than the average person would use on a cycle. In fact, I have never, in my years of researching steroids and speaking with athletes, heard of anybody using 200mgs/day of Halotestin, Winstrol, or Dianabol. And, at the end of that study, In vivo, each rat still had liver enzyme levels that were within normal range!
In another study (Hartgens, 1996), 16 bodybuilders using steroids were compared to 12 bodybuilders who were not. Then the bodybuilders who had used steroids stopped taking them for three months, at which points, the researchers found that liver enzymes had returned to the same levels as the non users. After only 3 months!
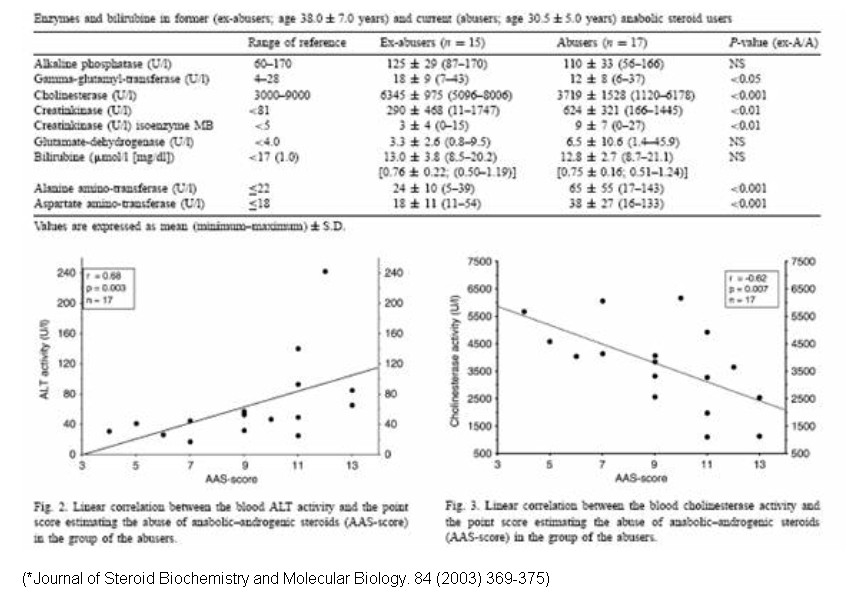
We can see from the chart below that ex-steroid users have totally normal liver enzymes one year after they stop using .in fact, for some liver enzymes, even the current users have normal scores!
Let's move on to some more useful studies. Take for example a 1995 study that showed the toxic effects of anabolic-androgenic steroids in primary rat hepatic cell cultures[3]. In this study the researchers used the following drugs and dosages:
As proof of the hepatoxicity they used Lactate dehydrogenase release, neutral red retention, and glutathione depletion to determine plasma membrane damage, cell viability, and possible oxidative injury, respectively.
What they showed was that the 17 alpha-alkylated steroids, methyltestosterone, stanozolol and oxymetholone, significantly increased Lactate dehydrogenase release and decreased neutral red retention at the 1x10^-4M dosage for 24h. Both methyltestosterone and oxymetholone also showed depleted glutathione at the 1x10^-4M dosage after 2h, 6h and 8h treatments. In other words they increased liver activity. You may also note that the other, non-alkylated steroids showed no significant difference in any levels. All in all this not only shows that 17 alpha-alkylated steroids are directly “hepatotoxic”, but also non-alkylated steroids are note hepatotoxic at all. But is this a real measure of hepatotoxicity? There is yet to be any correlation between the increase of the above-mentioned measurement and “hepatotoxicity”. Obviously, high dosages of the 17 alpha-alkylated steroids are potentially dangerous, but upon closer inspection, the study reveals more.
Take a look, the researchers took cell cultures from the liversse of 60-day-old Sprague-Dawley rats. Not only are rat livers much smaller than human livers, but these were merely cultures. Furthermore, it was the 1x10^-4M concentrations that caused the most changes, but these are approximately 1 to a 1/3 of a full, daily human dosage -- at least for the 17 alpha-alkylated steroids. Even at the 1x10^-6M concentration, there were no significant changes observed. It's apparent that the levels of 17 alpha-alkylated steroids used were potentially toxic, but for a human to take the same amount would be insane. I'm guessing this could translate to maybe 4 grams every 24 hours or 28 grams a week if not more.
.jpg)
Another study ( Boada 1999), attempted to show the acute and chronic effects of stanozolol on the liver. In acute treatments of stanozolol, dosages not mentioned, both cytochrome P456 and b5 (microsomal enzymes) levels dropped after 48 hours, and then at 72 hours, levels significant increased. On the other hand, with chronic treatments, time or dosage not mentioned, these microsomal enzymes showed a decrease in levels. Researchers showed that both acute and chronic treatments resulted in "slight to moderate inflammatory or degenerative lesions in centrilobular hepatocytes", but the authors did not note true hepatotoxicity.
As for human studies, ( Dickerman,1999) researchers tried to prove that the hepatotoxicity of steroids is overstated. In this study, 15 of the participants were bodybuilders using self-administered steroid dosages and 10 were non-steroid bodybuilders. Serum data was compared to 49 patients with viral hepatitis, and 592 exercising and non-exercising medical students.
All of the bodybuilders showed increases in aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatine kinase (CK) while gamma-glutamyltranspeptidase (GGT) levels were in the normal range. In comparison, hepatitis patients showed increased ALT, AST, and GGT levels while the control exercising medical students showed increased CK levels. From this, the researchers suggested that it is the correlation between AST, ALT and GGT that shows true liver dysfunction. Keep in mind, we can only guess that the 15 steroid users were using 17 alpha-alkylated steroids, and we do not know what the dosages that were used., but common sense tells us the results are likely relevant.
Last but not least, a simple study (Hartgens,1996), showed the long term benefits after taking a 3 month break from steroids. 16 bodybuilders using steroids were compared to 12 bodybuilders that were not. After a three-month drug withdrawal, the researchers showed that levels of liver enzymes, types not mentioned, returned to the same as the non users. Again the dosages are left to the reader’s imagination and we can only guess that the 16 steroid users were using 17 alpha-alkylated steroids.
So what can we conclude from all of this? First off, 17 alpha-alkylated steroids are hepatotoxic in high dosages taken for a long time. On the other hand, short cycles and small dosages appear to be perfectly safe. I suggest that maximum dosages should be 50mg to 90mg per day. They should be cycled for perhaps 8 weeks at a time, and if needed a 3-month break from them should be used. Using the above-mentioned techniques, your liver can be healthy for a long time. Simply put, the hysteria surrounding “hepatoxic” steroids, is based mainly on folk lore.
Are all 17alpha alkylated steroids equally toxic, or are some worse than others?
Oxandrolone is a c17-alpha alkylated compound. This alteration protects the drug from deactivation by the liver, allowing a very high percentage of the drug entry into the bloodstream following oral adminstration. C17-alpha alkylated anabolic/androgenic steroids can be hepatotoxic. Prolonged or high exposure may result in liver damage. In rare instances life threatening dysfunction may develop. It is advisable to visit a physician periodically during each cycle to monitor liver function and overall health. Intake of c17-alpha alkylated steroids is commonly limited to 6 – 8 weeks, in an effort to avoid escalating liver strain.
Oxandrolone appears to offer less hepatic stress than other c-17 alpha alkylated steroids. The manufacturer identifies oxandrolone as a steroid that is not extensively metabolized by the liver like other 17-alpha alkylated orals, which may be a factor in its reduced hepatotoxicity. This is evidenced by the fact that more than a third of the compound is still intact when excreted in the urine. Another study comparing the effects of oxandrolone to other alkylated agents including methyltestosterone, norethandrolone, fluoxymesterone, and methandriol demonstrated that oxandrolone causes the lowest sulfobromophthalein (BSP; a marker of liver stress) retention of the agents tested. 20mg of oxandrolone produced 72% less BSP retention than an equal dosage of fluoxymesterone, which is a considerable difference being that they are both 17-alpha alkylated.
A more recent study looked at escalating doses (20mg, 40mg, and 80mg) of oxandrolone in 262 HIV+ men. The drug was administered for a period of 12 weeks. The group taking 20mg of oxandrolone per day showed no significant trends of hepatotoxicity in liver enzyme (AST/ALT; aminotransferase and alanine aminotransferase) values. Those men taking 40mg noticed a mean increase of approximately 30 – 50% in liver enzyme values, while the group of men taking 80mg noticed an approximate 50 – 100% increase. Approximately 10 – 11% of the patients in the 40mg group noticed World Health Organization grade III and IV toxicity according to AST and ALT values. This figure jumped to 15% in the 80mg group. While serious hepatotoxicity cannot be excluded with oxandrolone, these studies do suggest that it is measurably safer than other alkylated agents.
A little insight about relative toxicity
All oral steroids have a chemical bond that must be broken before the drug becomes bioavailable. This may be a bond at the 17th position, or even at the 1st position (methenolone, proviron). Now, some steroids, like winstrol, have an additional bond that adds to its toxicity. Most orals have either one or two chemical bonds that need to be broken down in order to render the steroid bioavailable.
Halotestin (fluoxymesterone) is the harshest on the liver is because halo incorporates a combination of three chemical bonds that are all toxic features.
The chemical name for fluoxymesterone is androst-4-en-3-one, 9-fluoro11,17-dihydroxy-1,7-methyl-,(11b,7b)-. So we see that it has 17A methyl(17alpha alkylated), which is indeed toxic. This is the bond that gives us the name 17 alpha-alkylated, and the one that is common among all orals.
The second arrangement that halotestin contains is 9-alpha fluorine (fluorine is used to protect substances from breaking down, as with sodium fluoride, etc). This version of Fluorine is also very toxic.
Lastly, Halotestin contains some of the 11-Hydroxyl Group which is must be enzymatically reduced to the corresponding 11-hydroxy derivative before becoming biologically active. This reaction is carried out by a distinct 11-hydroxysteroid dehydrogenase isozyme in the liver that operates in a reductive capacity.
This process alone is very toxic, not just to the liver, but to the kidneys also.
With Halotestin, androgenic activity is increased 10 times and anabolic activity increased 20 times over that of 17-a methyl testosterone. This gives you an idea of its efficacy, and why it is such a favorite for those looking for added strength.
All oral steroids are somewhat "liver toxic" or burden the liver in a dose-related manner, and my experience is that the actual toxicity is somewhat exaggerated. However, for patients on steroid therapy, I usually recommend injectable steroids to reduce potential wear on the liver. And while these aren't actually toxic in the truest sense of the word, I recommend "cycling" all therapeutically-used anabolic steroids, with breaks between cycles, to give the body a rest.
Oxandrolone seems to be the mildest oral steroid on liverenzym activity the how and what can be read in other sections of this article
Toxic Effects
What are the known toxic effects of oral steroids? By far the most common toxicity seen is intrahepatic cholestasis. In general, cholestasis is any condition where bile flow is stopped, and with oral anabolics it occurs within the liver. Normally, bile is released into the small intestine and where its main function is to aid the in the absorption of fats and fatlike substances. This stoppage prevents bile salts from being released into the bile duct, causing a buildup within the hepatocyte. This buildup can be toxic to the hepatocytes over time. Jaundice, a yellowing of the skin and eyes, is related to cholestasis. This occurs because bilirubin (a product of red blood cell breakdown), is normally eliminated through the bile. During cholestasis, this builds up and produces a yellowish color in the skin and eyes, and is a tell tale sign that something bad is happening. Jaundice is a rare thing to see except in newborn babies, and a healthcare professional should be sought out if you notice these symptoms. The type of cholestasis normally seen from oral steroid use is clinically categorized as ‘bland cholestasis' because there is no inflammation accompanying the cholestasis. This type of cholestasis is fully reversible upon cessation of the offending agent.
In addition to cholestasis, other reported toxic effects are peliosis hepatis and hepatic adenoma. Peliosis hepatis is the presence of blood-filled cavities in the liver. This is a rare occurrence, and the theory is that peliosis hepatis results because of liver blood outflow obstruction at the junction of sinusoids and centrilobular veins. What causes this? It is believed to be related to cholestasis, which causes growth (swelling) of the hepatocytes. In AAS users the obstruction may be due to the prolapse of hyperplasic hepatocytes into the hepatic venule wall. This is good news because this means if cholestasis can be prevented, so can peliosis hepatis.
Hepatic adenoma is mentioned several times in the literature as a possible effect of oral steroid use. The prevalence of this is extremely rare and seems to only occur after months or years of continuous use. It is very likely associated with prolonged cholestasis as well. In my opinion, it should not be a concern unless someone in your family has got this from an oral steroid (including birth control pills), and the real focus of safety should be on preventing cholestasis.
Liver Anatomy and Function
The liver has numerous important functions in the body, but its relevant functions for this article include drug metabolism and excretion, and secretion of bile salts and bicarbonate for digestion.
When orally ingested testosterone is absorbed in the small intestine it is transported to the liver via the portal vein. Here it is nearly 100% metabolized to a 17-keto steroid by the enzyme 17-hydroxy steroid dehydrogenase. This reaction is very rapid and only when high amounts of testosterone are ingested does the enzyme system get saturated, allowing some testosterone to get by unchanged. Other reactions are possible such as reduction of the ketone group on the 3 carbon, but these are not as important to toxicity of the steroid.
With 17-alpha alkylated steroids, this conversion from a 17-hydroxy to a 17-keto steroid is prevented. This is key, and if you remember anything from this article, remember the next few sentences. The main difference between 17-aa's and regular steroids is that one retains a free 17 hydroxyl group and one does not, when going through the liver. The reason that 17-aa are toxic is because the free hydroxyl is able to be conjugated with glucuronic acid, forming a D ring 17-glucuronide. It is not the 17-aa steroid that is liver toxic but rather its 17-glucuronide metabolite. So it's not that these steroids are harder to metabolize, but rather the way they are metabolized causes them to be toxic.
This fact goes for androgens as well as estrogens, 17-alpha alkylated and non 17-alpha alkylated steroids. Let me clarify that last part, normal steroids would be liver toxic if they did not get metabolized to the 17-keto steroid, so it may be more correct to say they are potentially toxic, but are not in normal use. An intravenous infusion of estradiol-17- glucuronide, testosterone-17-glucuronide or dihydrotestosterone-17-glucuronide would cause cholestasis just as oral methyltestosterone or ethinylestradiol does.
So what about the supposedly liver friendly oxandrolone? The following excerpt summarizes why it is liver friendly:
Unlike other orally administered C17alpha-alkylated AASs, the novel chemical configuration of oxandrolone confers a resistance to liver metabolism as well as marked anabolic activity. In addition, oxandrolone appears not to exhibit the serious hepatotoxic effects (jaundice, cholestatic hepatitis, peliosis hepatis, hyperplasias and neoplasms) attributed to the C17alpha-alkylated AASs.
I submit that its resistance to metabolism (17-glucuronidation) is the reason for its lack of toxicity.
So we now know 17-glucuronides are to blame for liver toxicity. Now let's examine how they cause cholestasis. Bile flow is regulated in two ways; bile salt independent flow, and bile salt dependent flow.
Bile salt independent flow is a passive process controlled mainly by the osmotic factors glutathione and bicarbonate. The exact mechanisms are not known, but it is known that biliary glutathione levels decrease significantly soon after a toxic steroid is administered. The total hepatic glutathione increases, which seems to indicate that glutathione transport to the bile duct becomes impaired. Bicarbonate transport to the bile is similarly impaired, but it is not due to impaired transporters, rather the gradient becomes diminished by some type of bicarbonate reuptake. These processes occur rapidly and are the first toxicities observed.
Bile salt dependent flow is an active process that is controlled by numerous membrane bound transporters. Specifically ATP bind cassette (ABC) transporters transport the bile salts from the blood into the hepatocyte (basolateral), and then from the hepatocyte to the bile (canilicular). The pumping of bile salts into the bile is the main force that drives bile flow, which is what we want for normal functioning. Although both basolateral and canilicular transporters are probably involved in hormone induced cholestasis, the most examined is the canilicular bile salt export pump (BSEP). Oral steroid glucuronides are known to interact with the promoter region of the gene for this transporter and to repress its expression. Besides repression of the gene, other factors may decrease the BSEP function as well. The transport of the BSEP from its point of synthesis to the canilicular membrane can be impaired in cholestasis, providing functional transporters in the wrong place within the cell.
Finally there is the genetic component. There is a great deal of genetic variation in ABC transporters among the population. Certain people are at a higher risk for developing cholestasis than others, and in the near future it will be possible for you to determine what genetic polymorphisms you have in your hepatic transporters. This should be very valuable information for anyone who is planning on taking a potentially liver toxic drug, whatever it may be. In the meantime, the best method for determining if you are at risk for cholestatic problems is to look to your family. Cholestatic conditions to be mindful of are cholestasis of pregnancy, progressive familial intrahepatic cholestasis, benign recurrent intrahepatic cholestasis, and Dubin-Johnson syndrome. Having close relatives which any of these conditions possibly puts you at a greater risk of having toxicity issues with oral AAS.
Sulphasalazine Can Reverse Liver Disease
Sulphasalazine, a cheap drug currently used for arthritis and IBD (inflammatory bowel disease) can reverse the scarring that comes with cirrhosis of the liver, say scientists from the University of Newcastle, UK. Doctors had always thought that fibrosis - scarring associated with cirrhosis - was irreversible. This new study on animals has shown the damage can be reversed with Sulphasalazine. In the UK, about 10% of the adult population have liver problems, mainly due to heavy drinking and obesity/overweight. The liver has hepatic myofibrobrlasts, these are cells that create scar tissue when the organ is injured. Hepatic myofibrobrlasts produce proteins which makes it more difficult to break down the scar tissue. In a healthy liver the scars gradually disappear and new healthy ones replace them. This does not happen when the liver tissue is diseased - and the scar tissue spreads. The scientists found that Sulphasalazine stops the hepatic myofibrobrlasts from producing the protein that protects the scar tissue cells. In other words, it helps the scar tissue to gradually melt away. If human trials show similar results, it could mean treating and-stage patients with Sulphasalazine rather than having them undergo a liver transplant. The scientists say they will start trials with heavy drinkers who no longer drink, but whose livers are not able to recover on their own. This drug could be a Godsend for alcoholics who have given up drinking. Even a seemingly small recovery of 10% can make a huge difference to the patient's general health and quality of life, say the researchers. The researchers say Sulphasalazine could halve the cirrhosis death rate in the UK. Treatment would cost £10 ($18.50) per week. Some Facts About Cirrhosis -- Responsible for 1.4 million deaths per year worldwide -- Responsible for 5,000 - 10,000 deaths per year in the UK -- Early stages are symptom free (so damage accumulates unnoticed) -- There is currently no cure. The only end-stage treatment is a liver transplant -- Most common causes are Hepatitis C (globally) and excessive alcohol consumption (developed countries) -- Scotland has particularly high rates among developed countries
The medicine, found by a team of doctors and scientists at Newcastle University, could become a potential alternative to liver transplants. Until now cirrhosis of the liver, caused by alcohol, obesity or the hepatitis C virus, was considered incurable in all but the rarest of cases. The only option for patients in the final stages of liver disease was to wait for a liver transplant. However, because of organ shortages many die while on the waiting list. Clinical trials of the drug Sulphasalazine are expected to begin in Britain next year. If these prove successful, the drug could be used to treat heavy drinkers, whose plight was recently illustrated by George Best, the former Manchester United footballer who died from liver disease last year. Sulphasalazine, which already has a licence to treat arthritis and inflammatory bowel disease, acts by preventing scarring from developing on the liver. Tests carried out in the laboratory and on animals have shown that the medication can even reverse damage already inflicted on the liver. The drug will initially be given to heavy drinkers who have given up alcohol, but too late for their liver to recover naturally. If this proves successful, the medicine will also be prescribed to alcoholics who continue to drink but show a determination to fight their addiction by reducing their intake. Professor Christopher Day, who heads Britain’s biggest team of liver specialists at Newcastle University, said: “If you stop a drinker with cirrhosis of the liver from drinking, the cirrhosis will still be there. Even though we remove the cause of the liver scarring, by this stage that is not enough. “The prospect is that you may be able to continue drinking. If the drug is not too expensive, I may say, of course we have to give these patients advice about drinking, but who are we to say, ‘Just because you are still drinking, we are not going to give you this drug’? I would be of the view that it should be tried in patients who are making an effort. “I would not give it to someone who continues to drink heavily every day, but if someone had cut down to three pints a night and was really trying, why not give him this drug that might help his liver recover?” Sulphasalazine may also relieve the ethical dilemmas of distributing scarce donated livers to the most needy and deserving. The decision to give Best a liver transplant was controversial because the late footballer continued drinking. Critics argued that the organ should have been given to someone whose illness was not self-inflicted. If the drug is not prohibitively expensive, it could be given to all liver disease patients, regardless of whether the damage had been caused by a congenital disorder or years of alcohol abuse. “This drug is not a finite resource, you are not stealing it from someone else — which is always a worry in public opinion. People are dying on the transplant list,” Day added. After years of heavy drinking or obesity, so many scars appear on the liver that it can no longer carry out its normal tasks such as storing essential proteins and vitamins while cleaning up toxic substances. The new use for the drug followed the discovery by Professor Derek Mann, a member of the team at Newcastle University, who identified the cells and proteins that may move the liver disease into reverse.
**information from diverse sources from the net**
- Login to post comments


